Electrochemical Behavior of Silver Electrode in Sulphuric Acidic Solution During Anodic Polarization
A. Bayesov 1, E. Tuleshova2, A. Tukibayeva3*, G. Aibolova2 and F. Baineyeva3
1D. Sokolsky organic catalysis and electrochemistry institute,
2Kh.A. Yassawi International Kazakh- Turkish University,
3M.Auezov South Kazakstan State University Department of “Chemistry”, M.Auezov South Kazakhstan State University, 5 Tauke-khan ave., Shymkent, 160000, Kazakhstan.
Corresponding Author Email : ainur_tukibaeva@mail.ru
DOI : http://dx.doi.org/10.13005/ojc/310403
Article Received on :
Article Accepted on :
Article Published : 24 Nov 2015
The results of studying the electrochemical behavior of silver in sulphuric acid solution during anodic polarization are given in this work. The investigation on studying electrochemical properties of silver has been carried out by the use of cyclic voltammetric method. It was investigated that complicated electrochemical reactions occur in aqueous solution of sulphuric acid using silver electrodes, in anodic area takes place the silver oxidation with formation of oxides and sulphate of silver.
KEYWORDS:anodic polarization; electrochemical behavior; potentiodynamic polarization curves; silver
Download this article as:| Copy the following to cite this article: Bayesov A, Tuleshova E, Tukibayeva A, Aibolova G, Baineyeva F. Electrochemical Behavior of Silver Electrode in Sulphuric Acidic Solution During Anodic Polarization. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Bayesov A, Tuleshova E, Tukibayeva A, Aibolova G, Baineyeva F. Electrochemical Behavior of Silver Electrode in Sulphuric Acidic Solution During Anodic Polarization. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=12711 |
Introduction
One of the promising methods currently in use, is the polarization by the AC of industrial frequency, which is the interest of researchers both in terms of oxide formation and destruction of passivated film. The peculiarity of the processes, occurring under the influence of industrial AC, is that when change the direction of current is possible to remove the oxide film, and create the conditions for an unhindered further dissolution of metal1.
The electrochemical processes involving precious metals, especially silver in connection with the increasing demand for its in various branches of technology and industry are interesting. Systematic studies, allowing establish regularities of electrooxidation of silver, followed by synthesis of its compounds, may lead both to an intensification of its production, and to solve problems of resource saving.
A considerable amount of research has involved silver electrodes in different media. The interest in the electroformation and electroreduction of insoluble silver salts in different electrolytes containing halides, hydroxides, carbonates, chromates, perchlorate, etc.2-8 and the particular aspects of their electrochemistry is based mainly on the relevance that these films have in galvanic cells, seawater batteries, second class reference electrodes and corrosion and passivation2. Moreover, the corrosion and passivation of Ag electrode in biological and physiological solutions has been made9,10.
There are a lot scientific works, devoted to the research of electrochemistry of silver.
For electrolytic silver plating are mainly used the solutions of complex salts, as the solutions of simple salts, for example, silver nitrate, and precipitates are obtained as coarse and loose. Cyanide solutions are widely used. It was developed ferrocyanide, pyrophosphate, iodide, sulphite electrolytes, which are used as substitutes of toxic cyanide electrolytes. Despite the fact that the silver is the first metal for the surface treatment, which was applied as the method of the electrochemical polishing, the problem of increasing the effectiveness of its anodic treatment is the actual to the present time. This is due to the advantages of electrolytic polishing before mechanical – less complexity in the processing of relief products and the ability to significantly reduce the dead weight loss of polished metal. Balmasov A.V. and his colleagues11 studied the effect of the addition of glycerol to the solution of potassium thiocyanate on the parameters of the process of silver electropolishing. The introduction an aqueous solution of the organic solvent extends the capabilities of control of the anodic process by changing the viscosity of the electrolyte and the diffusion coefficients of the reactants, the solubility of products of anodic oxidation, increases the effect of the surface passivation layer.
In the work12 by the anodic polarization curves were studied the effect of various factors on the kinetics and mechanism of anodic behavior of silver, as well as it was determined the composition and conditions of formation of a passivating anodic film on the anode of silver in solutions (KCN, KSCN, NaSCN, NH4SCN, KJ, Na2S2О3, NaJ, Na2SO3, NaF, KF, NH4F). It was established that the value of passivation current (Ip) depends on the concentration of solutions. This dependence can be quite diverse.
Nature of the electrolyte has a significant influence on the composition of the adsorbed film, formed on the surface of silver. Kozin L.F. and Daniltsev B.I.13 found, that in the process of dissolving the silver electrode surface was covered with a black adsorptive film in the ammonium- chloride-bromide solutions of thiosemicarbazide (TSC). It was shown by the method of Auger spectroscopy that the adsorption film, formed on the surface of silver is its hydrosulfides and sulfides.
The authors14 formulated the laws of dissolution of silver in acidic tiocarbamide electrolytes. It was established, that on the kinetics of the process has strongly influence the impurity of molecules H2S, formed at the introduction to the solution of Na2S, or accumulating in the electrolyte with the passage of time after its preparation. Catalytic effect is enhanced by growth of the duration of contact of the electrode with the solution up to the experiment began or by increasing the concentration of sulphide ions. It is shown by the experiments with the process of updating the surface potential sweep, that the catalytic effect is due to the adsorption of sulfide ions at the interface.
The kinetics of dissolution of silver oxide in the solution of KOH was studied by authors15. It was established, that silver oxide is dissolved in this solution under the laws of diffusion kinetics.
The electrochemical behavior of the silver electrode was studied by the method of triangular voltage pulses. At this silver oxidation process occurs at potentials more positive than 0.25-0.3 V. The chemical reduction of silver electrode, as well as electrochemical reduction significantly changes its electrochemical properties. Using a vacuum-electrochemical method for the preparation of the electrode, it was possible to ascertain the phenomenon of penetration of chemisorption of oxygen into the silver. The presence of oxygen has a significant influence on the adsorption of hydrogen16.
Two-stage oxidation are observed at the anodic polarization of silver in the solution an alkali by photovoltaic polarization and polarization curves methods, each of which the oxides of silver with different composition are formed: silver oxide (I) at the first stage and silver oxide (II) on the second. In both stages of oxidation at certain values of the anodic oxidation potential is terminated because of the passivation of silver17.
It was revealed a relationship between the characteristics of electrochemical processes for the case of separating of silver from chloride melts by the authors in the work18.
In the process of cathodic separation of silver from water-soluble thiourea complexes the polarization has basically concentration character19. For practical use of cathodic deposition of silver are recommended the working temperature of 40-50°C, the current density of 0,5-2 a/dm2, pH of solution is 2.
Comparative studies of the electrochemical behaviour of Ag in aqueous NaCl, NaBr and NaI solutions of various concentrations ranging from 0.1 M to 1.0 M have been made by means of cyclic voltammetry and chronoamperometry techniques, complemented with SEM observation2. The cyclic voltammograms of Ag in the three halide solutions are nearly similar and characterized by the occurrence of a broad anodic peak (AI) corresponding to the electroformation of AgX film (X- = Cl- , Br- , I- ) and a sharp cathodic peak (Cl) corresponding to the electroreduction of AgX to Ag.
A simple and inexpensive, single step synthesis of silver nanoparticles was achieved using sodium citrate both as a reducing and capping agent. Silver nanoparticles were synthesized by injecting sodium citrate solution into the solution of silver nitrate at high temperature. The synthetic process was carried out in aqueous solution, making the method versatile and ecofriendly20.
In the previous work we researched the electrochemical behavior of silver in sodium sulphate solution during anodic polarization21. The investigation on studying electrochemical properties of silver has been carried out by the use of cyclic voltammetric method. It was investigated that complicated electrochemical reactions occur in sodium sulphate solution using silver electrodes, in anodic area takes place the silver oxidation with formation of oxides and sulphate of silver, and in cathodic area the oxygen compounds and silver sulphate regenerate.
In this regard, the study of anodic dissolution of silver is relevant and timely.
In this work we present the results of the investigation of kinetic regularities of electrooxidation – reduction reactions of silver in acid solutions by the cyclic potentiodynamic polarization curves.
Material and Methods
We have detected anode-cathode and cathode-anode cyclic potentiodynamic polarization curves with a potentiostat SVA-1BM. The main polarization voltammetric curves were recorded by a scan rate of 10 mV/s and recorded on a flatbed XY potentiometer H 301/1. The silver rod is used as the working electrode, its working surface is end portion, and the surface area is 4 mm2.
The measurements were performed in a three-electrode cell in respect to silver chloride electrode (E° = +0,203 V) and platinum is used as an auxiliary electrode.
Mainly, voltammograms were obtained in temperature intervals of 20-70°C (293-343 K), at a scan rate of potential of 5-100 mV/s. In order to clarify the mechanism of the processes, occurring during the polarization by alternating current, the electrodes were subjected to cyclic polarization, i.e. curves were recorded first in the direction of cathode-anode, and then the anode-cathode.
Thus, the change of direction of current was simulated in a certain degree, which takes place during the passage of the alternating current.
Measurements were carried out as follows: after updating the surface of electrode was turn on the potential scan with a certain speed in the range of 5-100 mV/s and the corresponding potentiodynamic polarization curve was recorded.
In order to conduct research at a constant temperature, the special electrolytic cell of mark YASE-2, installed in the thermostat of mark ITZH-0-03 was used.
Result and Discussion
Fig.1 shows the cyclic anode-cathode and cathode-anode polarization curves of silver electrode in 0.5 M sulphuric acid solution.
At displacement of potential on the anode direction in cyclic polarograms the peak of anodic (curve 1, maximum – “a”) of the oxidation current observes, due to the formation of silver ions:
Ag0 – е– = Ag+ Е= 0,799 В (1)
Next on the polarograms a sharp decrease of value of the current is observed that is connected with the salt passivation of the electrode surface according to the reaction:
2Ag+ + SO42- = Ag2SO4 (2)
At more positive potentials the current of releasing of molecular oxygen is observed:
2 Н2О – 4е– → О2 + 4Н+ (3)
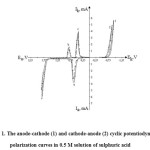 |
Figure 1: The anode-cathode (1) and cathode-anode (2) cyclic potentiodynamic polarization curves in 0.5 M solution of sulphuric acid Click here to View figure |
At this the surface of silver is covered with an oxide film of Ag2O, which can be seen visually.
At displacement of potential on the cathode direction in the potential range of maximum – “b” there is a reduction of oxide film:
Ag2О + 2Н+ + 2e– = 2Ag0 + Н2О Е = 1,173 В (4)
In the range of potentials “plus” 0,4V second maximum of current is observed, reflecting, apparently, the reduction of the formed silver sulphate (curve 1, a maximum “c”) by the reaction:
Ag2SO4 + 2е– = 2Ag0 + SO42- Е = 0,653 В (5)
When the potential of the “minus” 0б7 V and more values the release of hydrogen is observed:
2Н+ + 2е → Н2 (6)
The anode-cathode and cathode-anode cyclic polarization curves by the form are practically identical.
The concentration of sulphuric acid influences on the reaction of silver electrodissolution (Fig.2). By the dependence of lg i – lg C, a reaction order of the oxidation of silver was determined, which corresponds to the values of 0.21 and 0.47, which is characteristic for complex stage electrochemical reactions.
With increasing the concentration of sulphuric acid from 0.5 M to 2.0 M height of the current maximum of the oxidation of metal firstly increases and then decreases.
The curves, obtained during the temperature changes (20-70°C), are characterized by pronounced maximum of current, reflecting the oxidation of the metal.
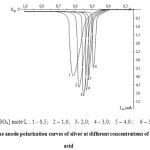 |
Figure 2: The anode polarization curves of silver at different concentrations of sulphuric acid |
By the temperature-kinetic rectilinear Arrhenius dependence apparent activation energy of the reaction of silver oxidation in sulphuric acid solution was calculated (Fig.3). It is shown that when over voltage of Δ E = 0,60 V an apparent energy of oxidation reaction of the metal is 10.06 kJ/mole. Based on these data we can assume that the oxidation of silver takes place in the diffusion regime.
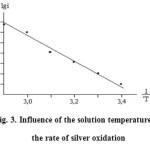 |
Figure 3: Influence of the solution temperature on the rate of silver oxidation |
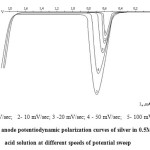 |
Figure 4: The anode potentiodynamic polarization curves of silver in 0.5M sulphuric acid solution at different speeds of potential sweep |
Fig. 4 shows the anodic polarograms of silver, obtained by speed of potential sweep of 5… 100 mV/sec. The height of the current of silver oxidation increases with an increase of speed of potential sweep. These data also indicate that the process takes place in the diffusion regime.
Based on the treatment of polarization curves by the method of Galius22, the diffusion coefficient and heterogeneous constant of the rate of electrochemical processes were determined.
In order to study the behavior of silver in conditions of the frequent periodic change of the direction of current, electrolysis was carried out by industrial alternating current. As preliminary experiments show, when polarization of two silver plate electrodes with alternating current at a current density from 200 to 1200 A/m2, the current efficiency of silver dissolution is not more than 4.8%23.
For the purpose of a possible strengthening of the process of dissolution of silver under the influence of AC, one of the silver electrodes was replaced by titanium.
It is known that an alternating current flows in two directions, at this a rapid change of the polarity of the electrodes is observed. When polarization by industrial alternating current in a second the change of polarity occurs 50 times.
The presence of valve properties of titanium promotes the flow of current only in the direction of the cathode and the effect of current “rectification” is observed in an anode. At the time, when the titanium electrode is in the anode half-cycle, an oxide film (TinOm), having valve properties is formed on its surface (24):
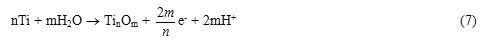
In the cathode half-cycle an oxide film may partially be reduced:
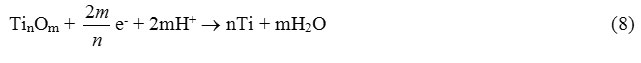
In the case of finding the titanium electrode in the cathodic half cycle the reduction of hydrogen ions will flow on it. At this moment the silver electrode is in the anodic half cycle and is oxidized to form ions by reaction 1.
Conclusion
Thus, we studied the electrochemical behavior of silver in the solution of sulfuric acid by cyclic potentiodynamic polarization curves. It is found that the concentration of sulfuric acid, temperature, the rate of potential sweep significantly influence on the anodic dissolution of silver. It is shown that the oxidation reaction of silver proceeds in the diffusion mode.
References
- Evans Y. R., Corrosion, passivity and protection of metals. Moscow –Leningrad, Metallurgy, (1941), 885.
- Hamdy, H.; Hassan, Magdy, A. M.; Ibrahim, Sayed, S.; Abd El Rehim, Mohammed & Amin, A. Int. J. Electrochem. Sci., 2010, 5, 278 – 294
- Ibrahim, M.A.M.; Hassan, H.H.; Abdel-Rehim S.S. & Amin, M.A. J. Solid State Electrochem., 1999, 3, 380
- Abdel-Rehim, S.S.; Hassan, H.H.; Ibrahim, M.A.M & Amin, M.A. Monatshefte fur Chemie, 1999, 130, 1207.
- Abdel-Rehim, S.S.; Ibrahim, M.A.M.; Hassan, H.H. & Amin, M.A. Can. J Chem., 1998. 76, 1156
- Abdel-Rehim, S.S.; Hassan, H.H.; Ibrahim, M.A.M & Amin, M.A. Monatshefte fur Chemie, 1998, 129, 1103.
- Ives, D.; Janz, G., Reference Electrodes, Academic Press, London, (1961).
- Amlie, R.F.; Honer, H.N.; Reutschi, P. J. Electrochem.Soc., 1965, 112, 1073
- Zielke, D.R.; Brady, J.M.; del Rio CE. J Endod., 1975, 1(11), 356-360
- Djokic, S.S.; Burrell, R.E. J. Electrochem. Soc., 1998, 145, 1426
- Balmasov, A.V.; Koroleva, E.V.; Lelin, S.Y. Protection of Metals, 2005, 41, 4, 386-389
- Yuzikis, P.A. Electrochemistry, 1987, 13, 11, 1388-1395
- Kozin, L.F.; Daniltsev, B.I. J. Phys. Chem., 2003, 77, 12, 2158-2164
- Beck, R.Y., Shuraeva, L.I., et al. Electrochemistry. 2007, 43, 3, 305-312
- Zavgorodnyaya, E.F.; Inyutina, T.Y.; Povarov, Y.M. Electrochemistry, 1987, 13, 3, 388-390
- Zhutaeva, G.V.; Shumilova, N.A.; et al. Electrochemistry, 1988, 4, 2, 196-198
- Oshe, E.K.; Rosenfeld, I.L. Electrochemistry, 1981, 7, 10, 1415-1417
- Polyakov, P.V.;, Burnakin, V.V. Electrochemistry, 1992, 8, 1, 26-29
- Nikolaev B.A., Harin A.N., Pluhotorenko V.I., Investigation of the process of cathode isolation of silver from their poorly soluble thiourea complexes. Studies on the electrodeposition and dissolution of metals. The collection of articles. Moscow, Nauka, (1984), 140-145.
- Guoqiang, Zhou; Wenying, Wang. Oriental J. Chem., 2012, 28, 2, 651-655
- Bayeshov, A.; Tuleshova, E.; Tukibayeva, A. Oriental J. Chem., 2013, 29, 1, 33-37
- Galius Z., Theoretical Foundations of electrochemical analysis. Moscow: Mir, (1974), 552.
- Tuleshova, E.Zh. Electrochemical Behavior of silver in aqueous solutions during polarization by alternating current. PhD thesis, Al-Farabi Kazakh National University, Almaty, (2009).
- Dospayev M.M., Baeshov A., Zharmenov, A.A., et al. About formation of copper oxide (II) during polarization of copper electrode by AC. Handbook of Chemistry, Almaty, (1988).

This work is licensed under a Creative Commons Attribution 4.0 International License.









