Drug - Excipient interaction studies of loperamide loaded in polsorbate 80 liposomes.
I. Sarath Chandran1 and Pichandy Muthu prasanna2*
1Research and Development Director , Rathnam Institute of Pharmacy , Nellore , Andhrapradesh , India.
2Research Scholar , PRIST University , Thanjavur ,Tamilnadu, India.
Corresponding Author Email: p_ra2000@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/310443
Article Received on :
Article Accepted on :
Article Published : 10 Nov 2015
Drug – delivery interaction studies were done for lopermide(Lp) loaded surfactant liposomes (LpLc) using FTIR ,DSC , XRD and SEM. Early researches have used Lp and liposomes for brain targeting programme but not have done any interaction studies .We have investigated presence of any drug degradative interactions with the excipient ( drug delivery – Liposomes) using FTIR , DSC , XRD and SEM analytical techniques. Our results suggests that presence of characteristic IR absorption peaks of Lp in LpLc in FTIR study confirmed absence of any drug interactions. Absence of Drug endothermic peak in DSC of LpLc indicates the amorphous nature of entrapped Lp . SEM ensured the absence of any undissolved drug or particles. XRD supports the existence of Lp in the LpLc without any drug interaction. In conclusion , drug loperamide (Lp) does not showed any degradative interactions with the carrier or excipient. Thus these studies supports the safety and efficient usage of Liposomes for Lp .
KEYWORDS:Loperamide; Liposomes; drug interaction studies
Download this article as:| Copy the following to cite this article: Chandran I. S, Prasanna P. M. Drug - Excipient interaction studies of loperamide loaded in polsorbate 80 liposomes. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Chandran I. S, Prasanna P. M. Drug - Excipient interaction studies of loperamide loaded in polsorbate 80 liposomes. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=12561 |
Introduction
Liposomes is a lipid based drug delivery used for loading both hydrophilic and lipophilic drugs1. Many researches were made for brain target due to its high lipid nature and its nano sized dimensions. Though liposome could deliver the loaded drug to brain , its lipid and water content has potential to degrade the loaded drug during the formulation and storage 2. Drug interaction studies with the carrier is required for effective and expected pharmacological response. Liposomes being a lipid based carrier can interact with the loaded drug via its degraded lipids.Lipid peroxidation is the oxidative degradation of lipids and liposomes, in particular, which are easily oxidised even in absence of specific oxidants. Lipid peroxidation of lipid bilayer membranes, especially for those containing cholesterol and phospholipids can affect both lipid membrane and the loaded drug3. Lipid peroxidation also results in the formation of conjugated dienes and malondialdehyde resulting in membrane modifications such as permeability, rigidity and conformational changes which can reduce the shelf-life of the liposomes and the drug4. Liposomal hydrolysis also provides a challenge for the loaded drug stability. Research has shown that hydrolysis of both saturated and unsaturated phospholipids are mainly catalysed by OH− and H+ of water present in the vesicles5 . In the presence of water, phospholipids can hydrolyse into fatty acids and lysophospholipids which are further hydrolysed into glycerophospho-compounds and fatty acids. The products of hydrolysis, namely lysophospholipids can increase the membrane permeability of the liposomes significantly, even at low concentrations and can degrade the loaded drug6. Factors that promote phase transition of the lipid bilayer membrane includes high temperature7 which may affect the loaded drug. These change in temperature can influence the drug degradation. Liposomes though a potent drug delivery needs to be investigated for any such drug degradation to ensure the expected therapeutic response.
Preformulation interaction studies such as Differential Scanning calorimetry (DSC) , Forier Transform Infrared Spectroscopy (FTIR) , X-Ray Diffraction Studies (XRD) and Scanning Electron Microscopy (SEM) supports any successive drug delivery design by ensuring the absences of any drug interaction with the delivery or its excipients. Loperamide – a potent opiod agonist which cannot pass blood bran barrier (BBB) was taken as a model drug for loading in surfactant liposomes for brain target scope as surfactant (polysorbate 80) in nanoparticle were reported in many earlier studies for successive brain target8. Phospholipids, cholesterol , water content of liposomes ,oxygen and polysorbate 80 present in the liposomes may affect the loaded loperamide by chemical degradation. Thus investigating these drug – liposomal interaction is needed for any successive drug delivery as drug – excipient interaction may either lead to low potency of drug or the drug delivery itself. Interaction studies such as FTIR , DSC , XRD and SEM establishes the status and stability of drug in the drug delivery and supports the retainment of both the potentials. In our study we have used loperamide loaded surfactant liposomes for drug – excipients interactions using DSC , FTIR , XRD and SEM.
Materials and methods
Preparation of Lp liposomes (LpLc):
Lecithin (Lec) and cholesterol (Ch) were used for liposome preparation with some modification of “Reverse Phase Evaporation Technique” as described by Szoka and Papahadjoupoulos et al9. In brief, lecithin and cholesterol (1:1) were dissolved in diethyl ether. A 5 ml Lp drug (2 mg/ml) in phosphate buffer saline (PBS) solution of pH 7. 4 were added . Vitamin E at 0.6 mol % were used to prevent lipid constituent’s oxidation. The formulation was emulsified using a homogenizer (Tenbroeck tissue grinder) at 5000 rpm for 20 minutes at a temperature of 50°C . The residual diethyl ether was evaporated using a vacuum evaporator (BUCHI EL 131 Rotavapor, Germany) under a reduced pressure (260 – 400 mm Hg) at 60 °C. The lipid gel so formed was collapsed and transformed to reach a fluid consistency by agitation using a vortex mixer. A 5 ml of warm PBS (pH7.4) was added to produces a suspension of multi lamellar vesicle liposomes (MLV) later sonicated using a microtip probe sonicator (Vibracell, sonics and materials, Inc, Danbury, CT) for 30 minutes at 40 % frequency producing a homogeneous dispersion. Nitrogen gas was flushed for 1 minute to remove trace oxygen. The liposome dispersion samples were kept at 4 °C and protected from light. 1 % w/v of polysorbate 80 was added to the liposomal suspensions and incubated for 30 min with constant stirring for preparing surfactant liposomes.
FTIR (Fourier Transform Infra red spectroscopy)
In order to get evidence on the possible interaction of Lp with liposomal constituents (Lc) at molecular level , Drug (Lp) and Loperamide loaded Liposomes (LpLc) in 1:1 molar ration were prepared in KBr (Potassium Bromide) disks and subjected to FTIR studies using Perkin Elmer Spectrum one, FTIR spectrophotometer (1600 series, Perkin-Elmer Inc, Norwalk, CT). The scanning range was from 450 cm−1 to 4000 cm−1 and the resolution was 1 cm−1 10.
DSC (Differential Scanning Calorimetry)
Physical Interaction studies between drug – Loperamide (Lp) and excipients (Liposomal constituents) (Lc) were studied by DSC. About 2mg of each sample Lp and LpLc were heated separately in a sealed aluminum pan(capacity – 4µl) under nitrogen flow (30ml/minute) at a scanning rate of 5oC /minute from 40ºC to 600ºC using Differential Scanning calorimeter (Spectrum one , Perkin Elmer , Model Sd 10 , Norwalk, CT).The empty aluminium pan was used as a reference. The heat flow as function of temperature was measured for each samples. Thermogram was then analyzed in comparison with the spectrum of drug and the excipients to assess the interaction of drug with the excipients.
XRD (X-Ray Diffraction study)
A Rigaku Ultima III X-ray diffractometer was used with a Cu-Kα target to determine the physical structure of Lp , liposomes and Lp loaded liposomes (LPLc). Approximately 250 mg of sample was used for the XRD analysis. The X-ray generator was operated at voltage 40 kV and current 100 mA. XRD patterns were obtained between 2θ angle range 10˚ – 60˚ with a scan speed of 2 deg/min.
SEM (Scanning Electron Microscopy )
Surface morphology of surfactant modified liposomal formulations were studied by Scanning Electron Microscope (JSM-6360, JEOL Electron Microscope, Japan) separately at various magnification range . A thin layer of sample (LpLc) were fixed on the carbon adhesive tape on an aluminium stub and excess sample removed. The stub was kept under vacuum overnight .The samples were then sputter coated with platinum under argon atmosphere at 180 mA for 1 minute using the autofine coater (JFC-1600, JEOL japan). The scanning electron micrographs were obtained using a Field Emission Scanning Electron Microscope, operating at 2 kV (JSM-6360, JEOL Electron Microscope, Japan). Picture of the liposome was taken by random scanning of the stub.
Results and Discussion
FTIR
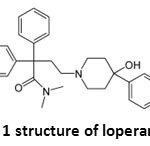 |
Figure 1: structure of loperamide Click here to View figure |
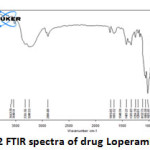 |
Figure 2: FTIR spectra of drug Loperamide Lp Click here to View figure |
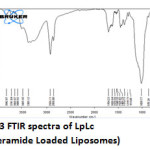 |
Figure 3: FTIR spectra of LpLc (loperamide Loaded Liposomes) Click here to View figure |
The IR spectrum (Fig 2 and 3) can be divided in to two regions. The left half above 2000 cm-1 has very few peaks and the right half below 2000 cm-1 of many peaks of varying intensities. Both Lp and LpLc IR spectrum showed similar characteristic absorption peaks11 ensuring the presence of same functional groups as follows. A very broad peak was obtained for both Lp and LpLc between 3300 and 3600 cm-1 indicating the presence of exchangeable protons (-OH ) stretching (ϑ). Just below 3000cm-1 (2800 -2900 cm-1) indicates presence of saturated carbons which confirms the presence of –CH ϑ in both Lp and LpLc. In the right half of the spectrum , below 2000 cm-1 which is a finger print region showed many peaks characteristic of many functional groups in Lp and LPLc IR spectrum and C-O ϑ is found in 1200 – 1250 cm-1. For C=C ϑ , C-Cl ϑ and C-N ϑ , the absorption peaks were in the range of 1660 -1710cm-1 , 870 – 873 cm-1 and 1150 – 1153 cm-1 respectively for both Lp- LpLc. Thus these characteristic absorption peaks study (Table 1) ensured most of the functional group peaks of Lp (drug) in the LpLc and this indicates absence of any drug excipient interactions and compliments liposomes to be a safer carrier for the drug loperamide.
Table 1: Functional groups and its respective absorption range of Lp and LPLc
|
Functional Group |
IR absorption range (Stretching vibrations – ϑ) (cm-1) |
Lp (cm-1) |
LpLc (cm-1) |
|
=CH |
3200 – 3400 |
3248.53 |
3385.12 |
|
-OH |
3300 – 3600 |
3548.51 |
3608.92 |
|
-NH |
3000 – 3700 |
3351.56,3563.58 |
3485.92,3669.97 |
|
-CH |
2800 – 2900 |
2896 |
2868.00, |
|
C=O |
1600 – 1650 |
1615.05 |
1626.64 |
|
C-O |
1200 – 1250 |
1204.81 |
1214.73 |
|
C=C |
1660 -1710 |
1665.52 |
1704.23 |
|
C-Cl |
870 – 873 |
873.07 |
870.23 |
|
C-N |
1150 – 1153 |
1152.75 |
1151.08
|
DSC
 |
Figure 4: DSC endotherm of Drug – Lp and LpLc (Drug loaded Liposomes) Click here to View figure |
Moreover , Loperamide which is a hydrophobic drug , partitions in the the lipid layer and gets close to the phospholipid and cholesterol leading to many possible interactions 12,13. Establishing any interaction between these Lp functional groups and the phospholipids ensures the safety and stability of the drug . Change in transition temperature of the liposomes with and without Lp ensures the interaction of the drug with the excipient. Thermograms of drug Lp , empty and Lp loaded Liposomes were shown in the Fig 4. Lp showed an endotherm at 228.93°C 14. DSC thermogram of empty liposomes showed a broad endotherm at 52.84°C corresponding to the lipid bilayer components – phospahtidyl choline and cholesterol15 .The broad thermogram between 50°C to 100 °C was due to its water content which evaporates during the heat process 16 . The Melting point of cholesterol and phospahtidyl choline was already reported at 148.27°C and 128.93°C respectively .But during liposomal formation , the cholesterol and phospholipids converts to gel and its heat enthalpy changes for its liposomal form. As liposomes, endothermic peaks were found to be 52.84° C which was similar to the earlier reported study 17 indicating the complete interaction of cholesterol with phospahtidyl choline. Lp endothermic peak at 2280C was absent in the Lp loaded liposomes indicating loss of drug’s crystallinity to amorphous nature and indicates its intercalation with the lipid bilayers resulting in shifting of the phospholipid peak (52.84°C) to 159.21°C. Thus by this DSC studies , it was evidenced that the Lp interacted well with the phosphoipid bilayer and also confirms any absence of any crystallized Lp in the formulation.
XRD
 |
Figure 5: XRD Drug (Lp), liposomes and Drug loaded liposomes (LpLc) |
XRD studies (Fig 5) were carried out to investigate the state of the drug (crystalline/amorphous) in the lipid carrier. XRD pattern of Lp showed numerous sharp, narrow and intense peaks, indicating its high crystallinity at 21.42°,36.34°,and 41.48°. But Lp loaded liposomes indicates low or absence of those sharp peaks 18 .
SEM
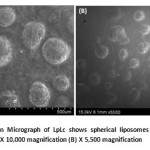 |
Figure 6: Scanning Electron Micrograph of LpLc shows spherical liposomes with absence of any drug particles or crystals (A) X 10,000 magnification (B) X 5,500 magnification Click here to View figure |
The liposomes entrapping the drug (Lp) resulted in complete spherical vesicles as shown in Fig 6 . Absence of drug crystals or undissolved particles ensured complete intercalation or entrapment of Lp in the liposomes.This SEM study confirmed no interaction of drug over formation of liposomes for its spherical vesicles in micrometer dimensions which showed LpLc in the size range of 500 µm to 1 mm.
Thus in our study , it was confirmed by FTIR , that Lp in the liposomes (LpLc) does not showed any loss or change in its functional group thus retaining its chemical nature. DSC and XRD ensured the presence of drug as amorphous form ensuring its complete intercalation with the lipid bilayers .Our SEM study confirms presence of spherical micro sized liposomes and complete absence non spherical liposomes or any undissolved drug particles.
Conclusion
Our study concludes the suitability of liposomes for the drug loperamide supporting the existence of drug in the carrier .Though Lipid degradation has potent enough to degrade any loaded drug , our study ensured the absence of any such degradation . Thus similar drug interaction studies need to be done for any drug and its respective carrier before evaluating its pharmaceutical scope.
References
- Langner, M.; Kral, T.E, Pol J Pharmacol. 1999, 51(3), 211-22.
- Lai,F. ; Fadda, A.M.; Sinico ,C. Expert Opin Drug Deliv. 2013, 10(7),1003-22.
- Maherani, B.; Arab-Tehrany. E.; Mozafari, M.R. Curr Nanosci. 2011, 7, 436–452.
- Vemuri, S.; Rhodes, C.T. Pharm Acta Helv. 1995, 70, 95–111.
- Mustafa, G.;Daan Crommelin, J.A.. J Chromatogr A. 1991, 585, 239–246.
- Crowe ,J.H.;. McKersie, B.D.; Crowe, L.M.Biochim Biophys Acta. 1989, 979, 7–10.
- Fernandez , M.S.; Gonzalez-Martinez , M.T.; Calderon, E. Biochimica et Biophysica Acta. 1986, 863,156–164.
- Alyautdin, R.N.; Petrov, V.E.; Langer, K.; Berthold, A.; Kharkevich, D.A.; Kreuter, J Pharm Res. 1997, 14(3), 325-8.
- Szoka, F. Jr, Demetrios Papahadjopoulos. Proc Natl Acad Sci U S A. 1978, 75(9), 4194-8.
- Robert M. Silverstein and Francis X Webster., Spectrometric identification of organic compounds, 6th edn, 136, John wiley and sons, Chap 12 New Jersey,(1970).
- Modern spectroscopy ,4th edition, J.Michael hollas , john willey and sons, 137 -151.(2004).
- Stamp, D.; Juliano, R.L.; Can. J. Physiol. Pharmacol.1979, 57, 535.
- Harika, C.; Palash, D.; Rahul, C.; J Pharma Biomed Sci. 2011,5(27),1-9
- Klaus Florey .,Analytical profiles of drug substances,Vol 19 ,Academic Press Inc.,UK p.no 345 – 355. (1990)
- Nagarsenker, M.S., Joshi, A.A.Drug Dev Ind Pharm. 1997, 23(3), 1159-1165.
- Ohtake, S.; Schebor, C.; Palecek, S.P.; de Pablo, J.J. Biochimica et Biophysica Acta. 2005, 1713, 57- 64.
- Scherphof, G.L. Ann N Y Acad Sci. 1985, 446(2), 368–84.
- Bruno C.hancock; Michael parks .Pharm Res . 2000 , 17(4) , 397 –404.

This work is licensed under a Creative Commons Attribution 4.0 International License.









