Conditioning of spent ion-exchange resins followed by solidification in the alkali-slag long-lived matrix with an increased level of filling with resins
Svetlana Nikolaevna Skomorokhova, Artem Nikolaevich Nikolaev, Elena Mikhaylovna Trifanova, Ivan Vladimirovich Sitnikov and Elena Aleksandrovna Grushicheva
Joint Stock Company "State Scientific Centre of the Russian Federation – Institute for Physics and Power Engineering named after A. I. Leypunsky" (JSC “SSC RF – IPPE”) 1, square Bondarenko, Obninsk, Kaluga region, 249033
DOI : http://dx.doi.org/10.13005/ojc/310439
Article Received on :
Article Accepted on :
Article Published : 23 Dec 2015
The possibility for spent ion-exchange resins (IER) of intermediate specific activity to be solidified in alkali-slag (geocement) water-resistant matrixes with an increased level of filling with resins was studied. Comparative tests of the IER immobilization process were done for justifying the most technologically effective matrix material. We used three different alkali-slag cementing systems and the prepared simulated pulps of IER with the specific activity of 3×108 Bq/L, saturated with 137Cs radionuclide. The manufactured samples of the alkali-slag compounds, filled with IER at the level of 24-27% by weight, meet the regulatory requirements set in NP-019-15 code and feature better working quality parameters (mechanical strength: 5-14 MPa, leaching rate of 137Cs, Na, Ca: <2×10-4 g/cm2∙day on the 7th-10th day, mechanical strength of compounds rises by the factor of 1.2-1.5 after immersion tests). The incorporation of the spent IER in the most technologically effective alkali-slag matrix makes it possible to decrease the cementing material consumption by the factor of 2.4 in comparison with Portland cement and by the factor of 1.3 in comparison with the known slag binders, while a compound with better quality parameters is produced. The research was done with the support of the Russian Ministry of Education and Science (unique identifier of the applied research studies - RFMEFI57915X0101) for justifying a new energy-efficient and resource-saving technology of reprocessing the spent IER-containing waste.
KEYWORDS:ion-exchange resins (IER); radioactive waste (RAW); cementation; conditioning; alkali-slag binders; matrix material; quality parameters of compounds; radionuclide immobilization
Download this article as:| Copy the following to cite this article: Skomorokhova S. N, Nikolaev A. N, Trifanova E. M, Sitnikov I. V, Grushicheva E. A. Conditioning of spent ion-exchange resins followed by solidification in the alkali-slag long-lived matrix with an increased level of filling with resins. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Skomorokhova S. N, Nikolaev A. N, Trifanova E. M, Sitnikov I. V, Grushicheva E. A. Conditioning of spent ion-exchange resins followed by solidification in the alkali-slag long-lived matrix with an increased level of filling with resins. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=13328 |
Introduction
NPP spent resins is a special type of heterogeneous radioactive waste of intermediate activity level. They are stored separately from other RAW categories and under a layer of water, and these conditions define, to a large extent, the approach to their reprocessing and conditioning [1].
At present the main methods of the spent IER reprocessing is direct solidification by cementation or bituminization. The cementation is a preferable one, as a rule, due to easier process operations, combustibility of the finished product, and high radiation stability of cement binding materials and cement matrix. Thermal methods of reprocessing followed by the incorporation of IER thermal destruction products in a water-resistant matrix rank high among the perspective developments in the field of the spent IER management. New processes of pyrolytic oxidation of various organic compounds and IER with the use of metals and their oxides as catalysts are studied. These studies are aimed at the improvement of engineering and economic parameters of the processes [1-5].
Along with the above stated, direct solidification methods (cementation, incorporation in a polymer matrix) are still topical, especially from the point of view of practical application [6].
A low degree of incorporation (usually not more than 10% by weight in terms of a dry resin for Portland cement matrixes) and a restricted storage time (absence of durability) of traditional Portland cement stones are referred to as the main problems of IER cementation. Specific features of the IER direct cementation are determined by tendency of the dehydrated ion-exchange resins to swell (due to the exothermic reaction of cement hydration). The formation of tiny cracks or general cracking of the cement compound structure, as a consequence of swelling, define poor mechanical properties and low water-resistance of the compound. Ion exchange of Сa2+ cation with the cement, interaction of binder’s components with some ions contained in IER (borates, sulfates, ammonia) are also referred to as the causes of the above mentioned problems [7]. The spent IER immobilization in a cement matrix is frequently connected with the problem of excessive water removal because the cement compound quality (water-resistance) is determined more by water/cement ratio than by IER concentration in the compound [8].
Experts believe that the problem of the rise of the level of the IER incorporation in a cement matrix can be solved by neutralization of the impacts that deteriorate physical and chemical properties of the finished product and hinder the implementation of the solidification process (for example, the exothermic reaction of cement hydration, setting time, and plasticity, in the course of mixing, of the pulp being solidified). IER amount that can be incorporated in a cement matrix is a function of the binder type and the water/binder ratio. The selection of a cement is an important factor that determines quality parameters of the solidified compound [9,10].
Due to the above mentioned, it is still topical to develop and introduce into practice new types of inorganic binding systems, to develop formulations of heterogeneous RAW cementation with the view of increasing the level of filling with IER, to improve quality parameters of the matrix material and its durability and, thus, to provide reduction of volume of the solidified compounds and enhancement of efficiency of using special storage facilities for the conditioned RAW.
Alkali-slag binders are a special type of binders. Their main advantage is good physical and mechanical properties and performance due to the synthesis of weakly basic hydrate phases of zeolite type as a part of stone – analogs of rock-building minerals [11].
Physical and chemical foundations of the theory of alkali-slag binders (alkali-slag cements and concretes) that had been developed in the middle of the XX century for the construction industry [12] have been used then for justifying the possibility of effective fixation of toxic and radioactive nuclides in the structure of these compounds with the view of isolating them from the biosphere [13].
Alkali-slag cements are produced by mixing ground granulated metallurgic slags with solutions of alkaline metal compounds. They are a special case of alkaline – alkaline-earth binders of hydration solidification. The main components of the alkali-slag cement are metallurgic granulated slags (blast-furnace, non-ferrous, steel-melting, ashes and slags of thermal power plants) and compounds of alkaline metals – sodium and potassium that demonstrate alkaline properties. The introduction of special additives of mineral origin – clay, burnt rock, glass-like rocks as well as clinker substances, including Portland cement clinker, makes it possible to produce alkali-slag cements with special properties. Specific features of the alkali-slag stone, which determine its good physical and mechanical and chemical properties, durability in comparison with the Portland cement stone is mineralogical composition of neoformations as well as its porous structure and structures of the “binder – filler” contact zone [14].
The alkaline compounds, added in the amounts that are optimal, play the role of not only slag activators, but act also as an independent component of an alkali-slag binding system. In the course of hardening of the cement grout the main products of the structure formation of alkali-slag binding systems – zeolite-like hydrate neoformations are formed as a result of consecutive processes of gelation, crystallization, and re-crystallization. They are analogs of water-resistant rock-building silicate minerals: alkaline and alkaline-earth hydroalumosilicates (Na,K)2O×Al2O3(2¸4)SiO2×2H2O and (Ca, Na2)O×Al2O3×nSiO2×mH2O, etc.; weakly basic hydrosilicates of calcium, e.g. tobermorates, (5¸6)CaO×6SiO2×nH2O. These processes take place during a long time and enhance gradually the compound resistance to natural media.
The operational advantages of the alkali-slag binders, as compared with traditional cements (strength, water impermeability, resistance to corrosion, frost-resistance, etc.), are consequences of special features of hardening products, which do not contain free lime and strongly basic hydrosilicates that are typical for traditional cement stone and deteriorate its properties.
In terms of strength properties, alkali-slag cement surpasses Portland, slag-Portland and aluminate cements. The alkali component makes strong plasticizing effect on alkali-slag cement, and, as a result, it features the reduced water requirement and the enhanced placeability. The heat rise that 2-3 times as low (as compared with Portland cement), high stability in the number of aggressive media, the enhanced frost-resistance and water impermeability are specific for alkali-slag cement [15].
The porous structure of alkali-slag cement (as compared with that of Portland cement) features the enhanced microporosity. The alkali component that is available in pores of different size and freezes at the lower temperatures than the porous liquid of the Portland cement stone determines gradual (not jump-like) character of freezing and the general enhanced frost-resistance of the alkali-slag stone. If the alkali-slag compound contains the filter, the alkali substances react not only with the slag but also with the filler’s surface. As the activity of this interaction increases, the degree of homogeneity of neoformations along the whole width of the contact zone rises and facilitates hardening [14].
National and international experts do research and development on the immobilization of various RAW using alkali-slag binders as a matrix material [16-20] with the view of optimizing formulations of solidification of various RAW including the spent IER.
It is the opinion of experts [21] that the application of slag cements is an illustrative example of using resource-saving technologies for solving environmental problems – radioactive waste is localized using man-made waste as a matrix material.
The experimental studies of physical and chemical properties and structure of the synthesized alkali-slag compounds (geocements) that have been performed earlier demonstrate that the application of a special formulation of the alkali-slag binding system for the solidification of various types of RAW provides production of a cement grout (in terms of plasticity, setting time and hardening) and a cement stone (in terms of correspondence to NP-019-15 code requirements) with good working properties. It has been shown that the fixation strength of main radionuclides of RAW (22Na, 137Cs, 90Sr, Т) of intermediate specific activity in alkali-slag cement compounds is determined by their incorporation, by means of isomorphous replacement, into the structure of water-resistant zeolite-like neoformations.
The experiments and developments on cementation were performed for the following types of RAW and their simulators incorporated in alkali-slag compounds:
- spent silicate sorbents (clinoptilotite, tripoli, bentonite, etc.) for concentration of radionuclides from liquid RAW with a low salt content;
- alkaline (30-50 NaOH by weight) and alkaline salt concentrates (400-500 g/L) – the liquid RAW formed in the course of washing BR-10 and BN-350 reactor equipment from sodium coolant;
- the concentrated liquid RAW (CLRAW) with the increased concentration of the organic components accumulated over 50 years.
Filling with the dry residue of concentrates reached up to 30% by weight in the synthesized alkali-slag compounds depending on composition and concentration of original liquid RAW, filling with organic sorbents – up to 35% by weight.
The quality parameters of the synthesized samples of alkali-slag stone meet the regulatory requirements specified for the cement compounds that are formed in the course of cementation of homogeneous and heterogeneous liquid RAW of intermediate activity level (NP-019-15 code) and can satisfy the requirements for their near-surface storage.
Durability of the compound being formed was predicted on the basis of the study of crystal structure and mineralogical composition of the samples of alkali-slag cement stones with different types of the incorporated RAW simulators. This property will make it possible to provide environmental safety of long-term storage of RAW [10,16].
Experiment
Three types of alkali-slag binding systems – based on PC-500 Portland cement, slag-Portland cement and granulated blast-furnace slag – were tested for the spent IER immobilization.
In our batches we used the granulated, finely ground blast-furnace slag of Novotulsky Metallurgical Plant (NTMK), in accordance with GOST (State Standard) 34-76-74, which contained (% by weight): 40.0 SiO2; 6.0 Al2O3; 44.0 CaO; 7.0 MgO; 0.5-1 Fe2O3; 0.2 TiO2. It possessed basicity properties (basicity index Ib=1.1) and specific surface of 0.4-0.95 m2/g.
Tripoli is a silicate sorbent from Zikeevsky deposit. It was used as an additive in all three binding systems. It is a fine-pored sedimentary rock with non-uniform mineral composition (opal-cristobalite, montmorillonite with quartz and clinoptilotite impurities). The finely dispersed silica (% by weight: 80-83 SiO2; 7-9 Al2O3; 1.5 CaO+MgO; 2-3 Fe2O3) with a high specific surface (≥ 101 m2/g) predominates in the tripoli composition.
The intended use of the components and additives, their concentrations in alkali-slag binding systems are justified by the following physical and chemical properties:
- alkali compounds act as slag activators and as an independent component of a binding system; their presence leads to the acceleration of dissolution and hydration processes in the binding system, facilitates strengthening of the alkali-slag stone structure. A necessary amount of NaOH in the composition of the binding system is calculated with regard for the requirement of equimolarity of alkali and alumina oxides in the binding system that is typical for the most alkali hydroalumosilicate minerals, which analogs are formed in the composition of the alkali-slag stone;
- additives of kaolinite, which falls into the group of non-swelling clay minerals (ion-exchange properties are generally inherent in them), help to enhance plasticity of the binding system and to deliver a necessary amount of Al2O3 to the binding system;
- additives of tripoli help to enhance strength and water-resistance of the cement compound due to binding of calcium oxide hydrate by an active silica in the clinker composition and formation of an additional amount of weakly basic calcium hydrosilicates – main carriers of high strength of silicate hydraulic binding systems; they also help to enhance stability of the cement grout by improving its capability to retain water.
The solidification process was tested using simulators of a pulp of spent resins of liquid RAW of a nuclear power plant (NPP) in the form of a mixture of KU-2-8 (50%) and AB-17 (50%) ion-exchange resins. As it is known, they are co-polymers of sterol and divinylbenzene with functional groups of SO3Н (KU-2-8) and –N(CH3)3OH (AB-17).
Standard procedures for the IER transition into H+-form (KU-2-8) and OH–-form (AB-17) followed by the transition into other salt forms by processing a cationite or an anionite with the saturated solution of the corresponding salt were taken as the basis for making simulators of the spent IER. Mixtures of ionites with the pre-determined ratios (Table 1) were prepared for making simulators of “low-active” and “high-active” spent IER of NPPs.
The incorporation of alkali components as NaOH hydroxide (in accordance with GOST 4328-77) or as sodium silicate solution (liquid sodium glass in accordance with GOST 13078-81 with silicate index Is = 1.5-3) into alkali-slag binding systems has been substantiated by the known data and the results of the studies (that have been performed earlier by the authors) of the application of alkali-slag cements (geocements) as perspective binders for the immobilization of various RAW [10,14-16].
Table 1: The composition of simulators of NPP’s spent IER
|
Name of a simulator of NPP’s spent IER
|
Simulator components |
Chemical form |
Ratio of KU-2 and AB-17 ionite volumes
|
Concentration of components in the total volume, % by volume
|
|
|
Simulators of “low-active” IER |
KU-2-8 AB-17-8 |
H+-form OH–-form |
1:1 |
25 |
|
|
KU-2-8 AB-17-8 |
Na+-form NO3–-form |
1:1 |
75 |
||
|
Simulators of “high-active” IER |
KU-2-8 AB-17-8 |
H+-form OH–-form |
1:1 |
10 |
|
|
KU-2-8 KU-2-8 AB-17-8 |
K+-form NH4-form |
1:1 |
1:1 |
90 |
|
|
BO33–-form |
|||||
In accordance with the procedure of batching the cement compound simulators of the spent IER are mixed under the pre-determined conditions with components of a binding system made of blast-furnace granulated slag or Portland cement, or slag-Portland cement with mineral additives and an alkali component solution in the ratio that corresponds to the composition of the cementing formulation being tested; and they are mixed until a homogeneous cement grout of normal density is formed.
The first group of batches was made on the basis of Portland cement (M PC-500 grade in accordance with GOST 31 108-2003) with additions of tripoli and an alkali component. The second group of batches was made on the basis of the finely ground granulated blast-furnace slag produced by “NTMK” in the mixture with kaolin (in accordance with Technical specifications (TS) 5729-10-40705684), tripoli and an alkali component. The third group of batches was made on the basis of slag-Portland cement (up to 70% by weight of slag), which contained the finely ground granulated blast-furnace slag produced by “NTMK” and Portland cement (PC-500) in the ratio of 1.5:1 with the addition of tripoli and an alkali component.
Simulators of the spent IER that had been saturated previously with 137Cs radionuclides up to the specific activity of 3×108 Bq/L using CsNO3 solution with the activity of 8.109 Bq/L were used for making cement samples for testing water-resistance by 137Cs leaching rate. The activity of the manufactured cement samples was 2-2×6.105 Bq/g.
The cement solution temperature, its density (by the volume-weight method), cement solution spreadability (in accordance with GOST 310.4-81), and the cement solution setting time (in accordance with GOST 310.3-92) were measured during batching.
Standard dismountable moulds with the mesh size of 20×20×20 mm were used for making samples of cement compounds that were subject to tests for mechanical strength, water-resistance, and impermeability to water (immersion tests); standard dismountable moulds with the mesh size of 70×70×70 mm were used for tests for resistance to thermal cycles in accordance with GOST 10060.0-95.
The total weight and the composition of the binding system (% by weight), the solution/binder ratio (S/B) (water/binder ratio (W/B)), the degree of the compound filling with IER (% by weight), and quality parameters of the solidified cement compound were recorded during manufacture of the cement compound samples. The cement stone samples were tested for mechanical compression strength and impermeability to water under (25±3)0C after their storage under the normal humidity conditions for 28 days.
The tests for mechanical compression strength were performed in accordance with GOST 310.4-81 by a certified procedure at “PRG-150” test press or “CD-4” universal hydraulic test machine with the following load ranges: 0–400, 0–2000, and 0–4000 kgf; its loading supports made it possible to center a sample and to compensate for non-parallelism of its butt ends. The measurement error of the ultimate breaking load is ±1%.
The value of the ultimate breaking load (P), expressed in kgf, and the value of cross-section area of the samples (Ssample), expressed in cm2, were obtained by measurements for each of two parallel samples.
The result of strength measurements (R) was calculated in MPa by the formula (1):
R = Р/Ssample, (1)
where Р is maximal value of breaking load, kN,
Ssample is sample’s cross-section area, cm2.
The impermeability to water of the samples was ascertained by a certified procedure in accordance with GOST R 52126-2003 by measuring the rate of 137Cs leaching to water. 137Cs was identified and its concentration was measured in the contact solutions by the semiconducting spectrometry method using a standard g-spectrometer. 137Cs measurement sensitivity was 5 Bq/100 mL. The measurement error did not exceed +5%.
The test results were calculated by the formula (2):
Rn = Аn/Аsp.F.tn, (2)
where Rn is leaching rate during the n-th period of time, g/cm2.day;
Аn is activity of the nuclide that has been leached over this time, Bq;
Аsp is specific activity of the nuclide in the original sample, Bq/g;
F is open geometric surface of the sample, cm2;
tn is duration of the n-th period of leaching, days.
In addition, in accordance with GOST R 52126-2003 (par.3.2), concentrations of the main components of the cement matrix – sodium and calcium ions were analyzed in the contact water samples, and the rate, at which they went out from the matrix, was evaluated.
The contact solutions were analyzed for sodium concentration by the acidimetric titration method with two indicators (phenolphthalein and methyl orange); the method sensitivity was 5×10-5 g of Na per an aliquot of the contact solution.
The contact solutions were analyzed for calcium concentration by the chelatometry method in accordance with GOST 3773-72; this method is based on the ability of calcium ions to form complex compounds with Trilon B at pH 10-12. The lower limit of calcium detection is 0.001 mg/sample.
The values of weight and weight concentration (mn/Ib) of a corresponding ion were used instead of nuclide activity values (Аn/Аsp) for calculating the rate of sodium and calcium leaching from the cement matrix to the contact solution.
Results and Discussion
The measurement results given in Tables 2-5 reflect qualitative characteristics of the samples of three groups of alkali-slag compounds that meet the regulatory requirements (NP-019-15 code), in terms of mechanical strength (≥4.9 MPa), and feature the compound filling with IER at the level of ≥21% by weight (in terms of the equivalent amounts of dry resins). It follows from the given Tables that on the basis of all three alkali-slag binding systems we synthesized the compound samples filled with resins at the level of 21-31% by weight, and these samples featured mechanical compression strength in the range of 5-14 MPa.
The analysis of the obtained results on the cement grout quality and mechanical strength of the samples enables us to conclude that the application of alkali-slag binding systems for the immobilization of spent ion-exchange resins makes it possible to achieve a higher level of the compound filling with IER, as compared with the known developments [3, 17].
As it follows from Tables 2-5, when the alkali component in the form of NaOH solution is replaced in the binding system’s composition with liquid sodium glass solution (with silica index Is=1.5), one may observe the enhancement of mechanical strength of the solidified samples (with the incorporated IER simulators) of all the synthesized alkali-slag compounds.
Table 2: The characteristics of the samples based on slag and kaolin with the IER simulated pulps
|
Sample No.
|
Compound composition, % by weight
|
S/B total, kg/kg |
Setting time, days |
Mechanical strength σcompr, MPa |
||||
|
Slag+Kaolin+Tripoli |
Water |
NaOH |
Liquid glass Is=1.5 |
IER (dry) |
||||
|
10 |
58.5 |
15.5 |
4.0 |
– |
22.0 |
0.71 |
< 1 day |
6.7; 6.1 |
|
68 |
54.1 |
10.3 |
4.6 |
– |
30.9 |
0.70 |
< 1 day |
5.9, 6.0 |
|
45 |
50.3 |
– |
– |
19.6 |
30.1 |
0.99 |
< 1 day |
12.6; 11.8 |
|
71 |
51.4 |
– |
– |
19.1 |
29.4 |
0.94 |
< 1 day |
13.3; 8.9 |
Table 3: The characteristics of the samples based on PC-500 M Portland cement with the IER simulated pulps
|
Sample No.
|
Compound composition, % by weight
|
S/B total, kg/kg |
Setting time, days |
Mechanical strength σcompr, MPa |
||||
|
PC+Tripoli |
Water | NaOH |
Liquid glass Is=1.5 |
IER |
||||
|
24 |
54.0 |
18.1 |
2.1 |
– |
25.8 |
0.72 |
< 1 day |
7.2; 5.8 |
|
23 |
56.1 |
18.2 |
2.1 |
– |
23.6 |
0.78 |
< 1 day |
5.5; 8.5 |
|
34-1 |
58.2 |
– |
– |
10.4 |
31.4 |
0.72 |
< 1 day |
5.0; 7.6 |
|
7U |
55.4 |
12.2 |
– |
5.1 |
27.3 |
0.81 |
< 1 day | 9.3; 12.1 |
|
44 |
54.7 |
9.2 |
– |
6.5 |
29.6 |
0.82 |
< 1 day |
11.0; 9.8 |
|
73 |
54.0 |
4.6 |
7.7 |
23.7 |
0.64 |
0.7-1 hour |
10.3;10.5 |
|
|
77 |
57.9 |
9.8 |
– |
7.6 |
24.7 |
0.64 |
0.7-1 hour |
11.3; 9.4 |
|
47 |
52.6 |
9.5 |
– |
9.2 |
28.7 |
0.90 |
< 1 day |
12.8; 7.7 |
Table 4: The characteristics of the samples based on slag-Portland cement with the IER simulated pulps
|
Sample No.
|
Compound composition, % by weight
|
S/B, kg/kg |
Setting time, days |
Mechanical strength σcompr, MPa |
||||
|
Slag+ PC-500 +Tripoli |
Water |
NaOH |
Liquid glass Is=1.5 |
IER |
||||
|
22 |
58.3 |
14.7 |
3.3 |
– |
23.7 |
0.65 |
< 1 day |
5.7; 4.2 |
|
27 |
57.8 |
17.5 |
3.3 |
– |
25.7 |
0.66 |
< 1 day |
Cracks |
|
43 |
47.5 |
8.5 |
– |
17.0 |
27.0 |
0.87 |
< 1 day |
8.9; 9.9 |
|
46-2 |
46.5 |
7.5 |
– |
17.5 |
28.5 |
0.93 |
2 days |
5.2; 11.4 |
|
74 |
56.3 |
8.7 |
– |
12.0 |
23.0 |
0.65 |
<1 hour |
14.4; 14.6 |
|
8У |
54.6 |
11.5 |
– |
9.7 |
24.3 |
0.70 |
<1 hour |
16.1; 11.7 |
|
34-R |
58.0 |
0 |
– |
16.2 |
25.8 |
0.62 |
0.7-1 hour |
9.3; 10.7 |
|
84 |
56.7 |
8.7 |
– |
12.0 |
22.6 |
0.67 |
0.7-1 hour |
10.5; 14.7 |
|
85 |
56.3 |
8.9 |
– |
12.1 |
22.7 |
0.66 |
0.7-1 hour |
10.7; 15.0 |
|
86 |
55.0 |
9.4 |
– |
11.7 |
23.9 |
0.70 |
0.7-1 hour |
8.1; 7.9 |
|
87 |
58.2 |
8.2 |
– |
12.3 |
21.3 |
0.62 |
0.7-1 hour |
11.7; 13.5 |
|
1-3 |
56.9 |
8.2 |
– |
12.1 |
22.8 |
0.65 |
0.7-1 hour |
10.9; 11.7 |
|
6-3* |
56.9 |
8.2 |
– |
12.1 |
22.8 |
0.65 |
0.7-1 hour |
11.4; 11.8 |
|
7-3** |
56.9 |
8.2 |
– |
12.1 |
22.8 |
0.65 |
0.7-1 hour |
9.0; 9.6 |
*Sample contains a simulator of low-active IER (see Table 1)
**Sample contains a simulator of high-active IER (see Table 1)
The results of immersion tests of the chosen alkali-slag samples manufactured on the basis of Portland cement and slag-Portland cement show that both types of the samples feature high water-resistance (Table 5). After holding them in water for 3-4 months the mechanical compression strength of the samples meets the regulatory requirements, and numerical values of it for the alkali-slag compounds based on slag-Portland cement rise, generally, by the factor of 1.2-1.5.
Table 5: The results of immersion tests of samples of the alkali-slag compounds based on Portland cement (PC) and slag-Portland cement (slag-PC)
|
Sample No.* |
Weight of initial sample, g |
Sample weight after holding it in water, g |
Sample composition |
Duration of holding** in water, days |
σ, MPa before tests (an average value) |
σ, MPa after holding in water
|
|
7 |
13.754 |
14.30 |
IER-PC |
100 |
6.87 |
8.0 |
|
34 |
14.224 |
14.34 |
IER-slag-PC |
123 |
9.16 |
9.85 |
|
43 |
13.709 |
13.69 |
IER-slag-PC |
114 |
9.4 |
9.98 |
|
44 |
14.817 |
14.94 |
IER-PC |
114 |
10.4 |
8.14 |
|
73 |
15.989 |
16.26 |
IER-PC |
100 |
10.36 |
21.1 |
|
74 |
14.930 |
15.24 |
IER-slag-PC |
100 |
14.4 |
23.1 |
* the samples shaped as cubes (20´20´20 mm).
** holding with the distilled water replacement.
The results of measurements of the rate of 137Cs, Na and Ca leaching from the studied samples, the character of leaching as a function of time of holding the samples in water (shown in Figures 1-4) reflect high water-resistance of the synthesized compounds.
The presented data demonstrates that the rate of 137Cs leaching from the samples with the incorporated IER that have been manufactured from slag-Portland cement is about 10-3-10-4g/cm2.day, but already from the second experimental point (that corresponds to 3 days during which a sample was held in water) this value becomes less than the permissible one: ≤1×10-3 g/(cm2.day). The rate of 137Cs leaching from the samples manufactured from PC-500 is less than the permissible value as well: ≤1×10-3 g/(cm2.day).
The results of measurements of the rate of sodium and calcium leaching from a series of cement samples, the character of leaching as a function of time of holding the samples in water and binding system’s composition (shown in Figure 3-4) demonstrate that the developed alkali-slag cement matrix based on slag-Portland cement with the incorporated spent IER features higher water-resistance (by one-two orders of magnitude) in terms of dissolution and yield of the matrix main components – sodium and calcium – to the distilled water in comparison with the alkali-slag matrix based on Portland cement.
 |
Figure 1: The rate of 137Cs leaching from cement stone samples with the simulated IER (24.3-24.7% by weight) based on alkali-slag binding systems (1 – sample No. 8U based on slag-PC ) (2 – sample No.77 based on PC-500) Click here to View figure |
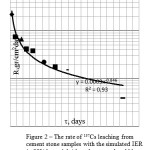 |
Figure 2: The rate of 137Cs leaching from cement stone samples with the simulated IER (~ 29% by weight) based on granulated blast-furnace slag with kaolin (sample No.71) Click here to View figure |
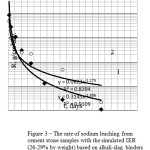 |
Figure 3: The rate of sodium leaching from cement stone samples with the simulated IER (26-29% by weight) based on alkali-slag binders (1 – sample No. 43 based on slag-PC) (2 – sample No.44 based on PC-500) Click here to View figure |
 |
Figure 4: The rate of calcium leaching from cement stone samples with the simulated IER (26-29% by weight) based on alkali-slag binders (1 – sample No.43 based on slag-PC) (2 – sample No. 44 based on PC-500) Click here to View figure |
The alkali-slag binding system based on the granulated blast-furnace slag with the addition of kaolin (geocement) makes it possible to synthesize the compound samples filled with IER at the level of ≥ 21% by weight. The solution of geocement mass features the enhanced plasticity. The grout was set and a necessary strength that was sufficient for demolding the samples was achieved over less than 1 day. As it follows from the data given in Table 2 and Figure 2, the samples manufactured on the basis of the geocement binding system meet the regulatory requirements specified for mechanical strength and water-resistance, and are characterized with the values of 5.9-13.9 MPa and <10-4g/cm2.day (137Cs leaching rate) already from the second experimental point (that corresponds to 3 days during which a sample was held in water). The properties of geocement compounds with various incorporated RAW of sorbents were described and analyzed in detail in paper [10].
The application of alkali-slag binding systems based on Portland cement and slag-Portland cement for the spent IER immobilization is of interest as more feasible and cost-effective version.
As it is known, slag-Portland cement that is more universal (as compared with pozzolanic cement) and cost-effective hydraulic binder is applied for RAW solidification increasingly frequently. Due to active mineral additives (blast-furnace granulated slag, natural hydraulic additives of tripoli, diatomite) slag-Portland cement features the enhanced water-resistance and resistance to sulfates and has satisfactory airproof and frost-resistant properties [1].
The ideas exist [14] that durability of alkali-slag compounds is predicted proceeding from special features of the structure formation process. Zeolite-like hydrate neoformations – analogs of water-resistant rock-building silicate minerals are formed through this process in the structure of an alkali-slag stone. These processes take place over long time and gradually enhance the compound resistance to natural media.
As it follows from the data given in Table 6, mechanical strength of the samples made of slag-Portland cement (PC-500 mixed with blast-furnace granulated slag) with the incorporated simulated pulps of IER after storage under normal humidity conditions over 5 years enhanced for almost all tested samples.
Table 6: The change of mechanical strength of the samples made of slag-Portland cement with the IER simulated pulps in case of long-term storage
|
Sample No. |
IER concentration in the compound, % by weight |
S/B, kg/kg |
Mechanical strength σcompr., MPa |
|
|
After storage over 28 days |
After storage over 5 years |
|||
|
43 |
27.0 |
0.87 |
8.9; 9.9 |
10.6 |
|
85 |
22.7 |
0.66 |
10.7; 15.0 |
11.6 |
|
86 |
23.9 |
0.70 |
8.1; 7.9 |
8.0; 10.7 |
|
87 |
21.3 |
0.62 |
11.7; 13.5 |
13.0 |
|
1-3 |
22.7 |
0.65 |
10.9; 11.7 |
10.9; 10.2 |
|
6-3* |
22.8 |
0.65 |
11.4; 11.8 |
12.4; 10.6 |
|
7-3** |
22.8 |
0.65 |
9.0; 9.6 |
10.7 |
*Sample contains a simulator of low-active IER (see Table 1)
**Sample contains a simulator of high-active IER (see Table 1)
The enlarged batches were made in a test mixer of 18L in volume for experimental tests of the formulations. It enabled us to manufacture cement solutions and solidified compounds of 5.5kg and 7.3kg in weight; and they included the spent IER in the amount of 27.3% and 24.3% by weight, correspondingly (Table 7).
The quality evaluation of the solution-cement mass in the course of batching showed satisfactory uniformity, absence of lumps or residual dry impurities in the main bulk of the solution-cement mass as well as in the near-wall sections of the mixer in all batches.
It has been found out that a temperature rise (as compared with the initial one) was not observed during batching of the solution-cement mass using the well-tried formulations as well as during hardening of the enlarged samples. The setting time of the solution-cement mass made in accordance with the well-tried formulations did not exceed 1-2.5 hours.
The characteristics of the samples manufactured from slag-PC as well as from PC-500 meet the set quality criteria related to mechanical compression strength and water-resistance (Table 7).
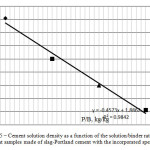 |
Figure 5: Cement solution density as a function of the solution/binder ratio in the cement samples made of slag-Portland cement with the incorporated spent IER |
Proceeding from the experimental results, the alkali-slag binding system based on slag-Portland cement, that contains the finely ground granulated blast-furnace slag in accordance with GOST 34-76-74, Portland cement (M PC-500 grade), tripoli, alkali component as sodium silicate solution in accordance with GOST 13078-81 with silica indexIs=1.5 (liquid glass, D=300 g NaOH/liter), has been recommended as the most perspective system for direct solidification of the spent IER of NPPs.
The obtained data enabled us to make calculation assessment of the consumption of an alkali-slag binding material for the immobilization of the spent IER of NPPs per a 200L barrel; the filling factor was 0.9. It follows from the calculation results that the consumption of binder’s dry component for the solidification of 100 kg of the spent IER’s pulp will be 170 kg including the finely ground granulated blast-furnace slag, Portland cement and tripoli as well as 30 kg of the alkali component as liquid glass solution with Is=1.5. Filling with resins reaches the level of 24.6-27.3% by weight (in terms of dry IER) or 58-70% by weight of the IER pulp in the compound in case when the spent IER are solidified in the alkali-slag binding system.
Therefore, the incorporation of the spent IER in the alkali-slag matrix makes it possible to reduce the binding material consumption by the factor of 2.4 in comparison with Portland cement (filling factor in terms of dry IER is 10% by weight) and by the factor of 1.3 in comparison with the known slag binders (filling factor in terms of dry IER is 18.2% by weight), while a compound with better quality parameters is produced.
Table 7: The results of making the enlarged batches in the test mixer
|
Characteristics of initial materials |
Batch 1 IER – 1.5 kg |
Batch 2 IER – 1.8 kg |
|
Compositions of binder’s components and additives
|
PC-500 + tripoli *Liquid glass Water |
Slag +PC-500 + tripoli *Liquid glass Water |
|
Cement solution characteristics |
||
|
S/B, kgг/kg |
0.81 |
0.70 |
|
Density r1, kg/dm3 |
1.77 |
1.57 |
|
Temperature, 0C |
35 |
29 |
|
Spreadability, mm |
90-95 |
90-95 |
|
Setting time, hour |
0.7-1 |
0.7-1 |
|
Cement compound characteristics |
||
|
Weight, volume
|
5.49 kg; 3.10 dm3 |
7.32 kg; 4.65 dm3 |
|
IER concentration, % by weight |
27.3 (IER) |
24.6 (IER) |
|
σ, MPa |
12.1; 9.3 |
9.9 |
|
σ, MPa after holding in water for 90 days |
8.0; 8.2 |
12.1;11.6 |
*- sodium liquid glass solution with Is = 1.5
Conclusion
The results of studies of how mechanical strength and water-resistance of alkali-slag samples meet the regulatory requirements of NP-019-15 and RD-95 10497-93 codes attest high quality of the alkali-slag compounds filled with IER up to the level of 24.6-27.3% by weight and manufactured by the developed formulations on the basis of Portland cement, slag-Portland cement, and blast-furnace finely ground granulated slag. Mechanical strength is ≥5-14 MPa, 137Cs leaching rate after 3 days of holding in water is (3.2-9.5×10–4) g/cm2×day for the solidified compounds with the incorporated IER. Calcium leaching rate is (1-6)×10–4 g/cm2×day after one day of holding in water.
It has been concluded from the tests of 137Cs, sodium, and calcium leaching from the alkali-slag samples that the compounds based on slag-Portland cement binding system with the incorporated spent IER demonstrate higher water-resistance (by one-two orders of magnitude) of the main matrix components – sodium and calcium – in comparison with the samples based on Portland cement binding system.
The results of sampling water-resistance tests demonstrated the enhancement of mechanical strength of the compounds after immersion tests by the factor of 1.2-1.5.
The quality analysis of the cement grout and the solidified matrices enables us to recommend the alkali-slag binding system based on the finely ground granulated blast-furnace slag and Portland cement (PC-500) at the ratio of 1.5:1 with the addition of the dispersed silica (of gaize-tripoli type under TS 21663-001-26127152-94, M-80 grade) and the alkali component as liquid sodium glass in accordance with GOST 13078-81 with silica index Is=1.5, as more technological system for solving the task – direct cementation of NPP’s IER on the basis of an alkali-slag binding system.
The obtained data enabled us to make calculation assessment of the consumption of a binding material for the immobilization of the spent IER of NPPs. This assessment demonstrated that the solidification of the spent IER to an alkali-slag matrix in accordance with the developed formulation made it possible to reduce the binder consumption by the factor of 2.4 in comparison with traditional inorganic binders and by the factor of 1.3 times in comparison with the known slag binders, while a compound with better quality parameters is produced.
Tests of mechanical strength of the alkali-slag samples after long-term storage (5 years) proved experimentally the enhancement of mechanical strength of the samples in the course of time.
The results of tests of quality parameters of the alkali-slag compounds with the incorporated spent IER as well as the results of the earlier performed studies of crystal structure and mineralogical composition of samples of alkali-slag cement stones with the incorporated various simulators of RAW demonstrate that the application of an alkali-slag binding system for the immobilization of NPP’s spent IER makes it possible to provide not only a higher level of filling with RAW components but also the improved quality of cement compounds and, above all, to provide their long-term storage, during which the compound strength and water-resistance rise in the course of time.
The authors acknowledge scientific consultations given by N.G. Bogdanovich, Cand.Sci. (Chemistry).
The research was done with the support of the Russian Ministry of Education and Science (unique identifier of the applied research studies – RFMEFI57915X0101) for justifying a new energy-efficient and resource-saving technology of reprocessing the spent IER-containing waste.
References
- International Atomic Energy Agency, 2002. Application of ion exchange processes for the treatment of radioactive waste and management of spent ion exchangers. Technical Reports Series No. 408 (DOC/010/408). Vienna.
- Yaroslavtsev, G.F., Reznik, A.A., Korchagin, Yu.P., 2005. Obrashchenie s radioaktivnymi otkhodami AES kontserna “Rosenergoatom”. Radioactive waste management. MNTK-2005. Proceedings of the 5th International Science and Practice Conference, pp: 3-14. Moscow: JSC VNIIAES.
- Yepimakhov, V.N., Oleinik, M.S., 2005. Vklyuchenie radioaktivnykh ionoobmennykh smol v neorganicheskie svyazuyushchie. Atomnaya Energia, 99, 3, 171-177.
- Bortnikova, M.S., Karlina, O.K., Pavlova, G.Yu., Semenova, K.N., Dmitriev, S.A., 2008. Konditsionirovanie shlaka, obrazuyushchegosya pri termokhimicheskoi pererabotke ionoobmennykh smol. Atomnaya Energia, 105, 5, 274-278.
- Ulyanov, V.V., Gulevsky, V.A., Martynov, P.N., Fomin, A.S., Shelemetiev, V.M., Sadovnichii, R.P., Niyazov, S.-A.S., 2012. Primenenie teplonositelei Pb i Pb-Bi v novykh tekhnologiyakh pererabotki tverdykh, zidkikh i gazoobraznykh sred. Izvestiya vysshikh uchebnykh zavedenii. Yadernaya energetika, 4, 102-109.
- Kiselev-Dmitriev, A.L., Masanov, O.L., Zakharova, K.P., 2001. Issledovanie protsessa otverzhdeniya otrabotavshih filtrmaterialov i shlamov Leningradskoi AES. “Safety, Efficiency and Economics of Nuclear Power Engineering”. A Book of abstracts of the Second International Science and Technology Conference, part II, p. 140. Moscow: VNIIAES.
- Chemichev, E., 1996. Peredovaya tekhnologiya vklyucheniya v tsement radioaktivnykh otkhodov. Razrabotki, provedyennye dlya kubovykh ostatkov i otrabotannykh ionoobmennykh smol. Proceedings of a Russian-French workshop, Obninsk.
- Polyakov, A.S., Zhikharev, M.I., Zakharova, K.N., Khimchenko, O.M. et al, 1985. Otverzhdenie zidkikh otkhodov srednego urovnya aktivnosti s ispolzovaniem neorganicheskikh vyazhushchikh. Atomnaya Energia, 58, 4, 249-252.
- Barinov, A.S., Varlakov, A.P., Gorbunova, O.A., Nevrov, Yu.V., Germanov, A.V., 2008. Novye tekhnologii tsementirovaniya RAO. Bezopasnost okruzhayushchei sredy, 3, 74-77.
- Konovalov, E.E., Bogdanovich, N.G., Skomorokhova, S.N., Myshkovsky, M.P. et al, 2006. Geotsementnyi kamen – ustoichivyi matrichnyi material dlya immobilizatsii radioaktivnykh otkhodov. Radiokhimiya, 48, 1, 74-77.
- Glukhovsky, V.D., 1989. Betony proshlogo, nastoyashchego i budushchego. Alkali-slag cements, concretes, and structures. Proceedings of the 3rd All-USSR Science and Practice Conference, vol.1 pp: 7-11, Kiev.
- Gelevera, A.G., 1994. Alkaline Portland and slag Portland cements. Alkaline cements and concretes. Proceedings of the First International Conference, v.1 рp: 173-179, Kiev, Ukraine: Scientific-Research Institute on Binders and Materials named after V.D. Glukhovsky.
- Krivenko, P.V., Skurchinskaya, Zh.V., Konovalov, E.E., 1994. Physical-chemical bases of radioactive wastes immobilization in a mineral-like solidified stone. Alkaline cements and concretes: Proceedings of the First International Conference, V.2. рр: 1095-1106, Kiev, Ukraine: Scientific-Research Institute on Binders and Materials named after V.D. Glukhovsky.
- Krivenko, P.V., 1990. Fyziko-khimicheskie osnovy dolgovechnosti shlako-shchelochnogo kamnya. Tsement, 11, 2-5.
- Skurchinskaya, Zh.V., 1989. Effektivnye puti sovershenstvovaniya svoistv shlakoshchelochnyh vyazhushchikh. Alkali-slag cements, concretes, and structures. Proceedings of the 3rd All-USSR Science and Practice Conference, vol.1 pp: 138-142, Kiev: Kiev Civil Engineering Institute.
- Skomorokhova, S.N., Bogdanovich, N.G., Konovalov, E.E., Korchagin, Yu.P. et al, 2011. Razvitie tekhnologii tsementirovaniya dlya konditsionirovaniya RAO. Proceedings of the VII International Nuclear Forum “Safety of Nuclear Technologies”, pp: 123-127. Saint-Petersburg, Russia: NOU DPO “TsIPK”.
- Oleinik, M.S., Yepimakhov, V.N., Trofimov, V.V., 2010. Vklyuchenie radioaktivnykh ionoobmennykh smol v shlakovoe svyazuyushchee. Radiokhimiya, 52, 6, 516-518.
- Perera, D., Vance, E., Kiyama, S., Aly, Z., Yee, P. (2007). Geopolymers as Candidates for Immobilisation of Low- and Intermediate-Level Waste. Mater. Res. Soc. Symp. Proc., 985, p.6.
- Hanna, J. V., Aldridge, L. P., Vance, E. R., 2001. Cs Speciation in Cements. Mat. Res. Soc. Symp. Proc. Vol. 663. Australian Nuclear Science and Technology Organisation, Menai, Australia: Materials Research Society. Materials Division, NSW 2234.
- Lutze, W., Gong, W., Pegg, I.L., 2009. Solidification of Low-Radioactive Waste Streams in Geopolymer Matrices. NAS Workshop on Waste Technology and Performance, p. 14. America Washington, D.C.: Vitreous State Laboratory, The Catholic University. 20064.
- Kozlov, P.V., Gorbunova, O.A., 2011. Tsementirovanie kak metod immobilizatsii radioaktivnykh otkhodov. Ozersk: RITs VRB FGUP “PO “Mayak”, p. 132.

This work is licensed under a Creative Commons Attribution 4.0 International License.









