Chemical profiling of Centella asiatica under different extraction solvents and its antibacterial activity, antioxidant activity
Supawan Rattanakom and Patchanee Yasurin*
Food Biotechnology Program, Faculty of Biotechnology, Assumption University, Bangkok 12040, Thailand Corresponding Author Email: patchaneeYsr@au.edu
DOI : http://dx.doi.org/10.13005/ojc/310480
Article Received on :
Article Accepted on :
Article Published : 11 Dec 2015
Centella asiatica (L) urban, synonym Hydrocotyle asiatica, is found almost all over the world. This plant is famous in Ayurvedic medicine and used in the management of central nervous system, skin and gastrointestinal disorder. Thus this research had been done to evaluate the effect of solvent extraction (Ethanol, Chloroform and Hexane) of C. asiatica on chemical profile, antioxidant activity and antibacterial activity against some foodborne pathogens. The result showed that all solvents (ethanol, chloroform and hexane) used in extraction showed antibacterial activity against Salmonella enterica Typhimurium U302, S. enterica Enteritidis, S. enterica 4,5,12:I human (US clone), Bacillus cereus and B. subtilis at 50mg/ml concentration. In antioxidant part, ethanolic extract gave highest phenolic content and FRAP value. The results also showed that different extraction solvent gave different chemical profile. Hexane extract C. asiatica showed lowest in both antibacterial and antioxidant activity. Ethanolic and chloroform extract of C. asiatica showed promising potential in both antibacterial and antioxidant activity.
KEYWORDS:Antibacterial activity; Antioxidant activity; GC-MS; Centella asiatica
Download this article as:| Copy the following to cite this article: Rattanakom S, Yasurin P. Chemical profiling of Centella asiatica under different extraction solvents and its antibacterial activity, antioxidant activity. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Rattanakom S, Yasurin P. Chemical profiling of Centella asiatica under different extraction solvents and its antibacterial activity, antioxidant activity. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=13266 |
Introduction
Background
Nowadays, there are increasing trends of using natural products. Interestingly, herbs are considered to be one of the alternatives consumers choose to use. Since prehistoric times, herbs were the basis for nearly all medicinal therapy until synthetic drugs were developed in the nineteenth century [1]. In Asia, people familiar with the used of herbal products and medicinal plants. Thus the availability of herbal products is high in both variety and volume. However the used of the medicinal plants or herbs still be based on traditional nostrum. In order to increase value of herbal products and medicinal plants, a knowledge-based scientific experiment should be conducted. C. asiatica (L) urban, belonging to the family Umbeliferae, is a common perennial herbaceous creeper abundant in moist areas and distributing widely in Asia [2]. C. asiatica is famous in Ayurvedic medicine for the treatment of leprosy, insanity, asthma, ulcers, eczema, skin tuberculosis, wounds, stomach aches, arthritis, varicose veins and high blood pressure [3]. Due to the benefits according in Ayurvedic medicinal practice of C. asiatica, this research had been done to evaluate the effects of solvent extractions of C. asiatica on chemical profile, antioxidant activity and antibacterial activity against some food borne pathogens. The expected outcome from this research would be to create a baseline data for a development of further advance research in C. asiatica utilization in various industries such as cosmetic industry, food industry, pharmaceutical industry, medical industry and agro industry.
Result and Discussion
Table 1: Antibacterial activity as inhibition zone (cm) of C. asiatica using different extraction solvents against different microorganisms
|
Microorganisms |
Inhibition zone (cm.) |
||
|
Ethanol |
Chloroform |
Hexane |
|
| S. enterica Typhimurium U302 | 1.108 ± 0.058 a,A | 1.125 ± 0.052 a,A | 0.983 ± 0.041 b,A |
| S. enterica Enteritidis | 0.983 ± 0.026 a,CD | 1.000 ± 0.031 a,B | 0.808 ± 0.024 b,C |
| S. enterica 4,5,12;i- human (US clone) | 0.942 ± 0.058 a,D | 1.175 ± 0.042 b,A | 0.875 ± 0.042 c,B |
| B. cereus | 1.033± 0.052 a,BC | 0.983 ± 0.041 ab,B | 0.941 ± 0.067 b,A |
| B. subtilis | 1.050± 0.084 a,AB | 0.983 ± 0.041 b,B | 0.875 ± 0.069 c,B |
Note: Different superscript within a row showed significant different at p < 0.05
Different superscript within a column showed significant different at p < 0.05
Antibacterial Activity of C. asiatica against Salmonella species
Three different solvents were used; 95% ethanol, chloroform and hexane in extracting antibacterial compounds from C. asiatica. From table 1, Ethanolic and chloroform extract were not significant different in antibacterial activity against S. enterica Typhimurium U302 with inhibition zone of 1.108 ± 0.058a and 1.125 ± 0.052a cm., respectively. However hexane extract showed significant lower in antibacterial activity with inhibition zone of 0.983 ± 0.041b. The results also showed that ethanolic and chloroform extract were not significant different in antibacterial activity against S. enterica Enteritidis with inhibition zone of 0.983 ± 0.026a and 1.000 ± 0.03a cm., respectively. However hexane extract showed significant lower in antibacterial activity with inhibition zone of 0.808 ± 0.024b cm. The results also showed that the antibacterial activity of ethanolic extract was significant different from chloroform extract against S. enterica 4,5,12;i- human (US clone) with inhibition zone of 0.942 ± 0.038a and 1.175 ± 0.042b cm., respectively. Hexane extract showed significant lower in antibacterial activity with inhibition zone of 0.875 ± 0.042c cm
At 32 mg/ml concentration of all extract didn’t show bactericidal effect (MBC). While at 8 mg/ml concentration inhibit effect can be seen (MIC). From the result in this research, it can be conclude that among all three solvents, chloroform seems to be most effective choice of solvent in inhibiting growth of three strain of Salmonella sp. compare to ethanol and hexane.
Similar to the work of Zhang et al. [4], they used broth culture method with the condition of incubated at 37ºC for 24 hour with 200 rpm agitation to test the antibacterial activity. At concentration of 40mg/ml, C. asiatica extract could reduce almost up to 1 log cycle of S. enterica Typhimurium compare to control. From the work of Mamtha et al. [5], they studied the effect of C. asiatica on enteric pathogens. From their results, at concentration of 100 mg/ml of crude C. asiatica ethanolic extract gave inhibition zone of 15 to 20 mm. on S. enterica Typhimurium. Lee and Varirappan [6] used antibacterial assay via disc diffusion method against 10 strains of food pathogenic bacteria and found out that essential oil and ethanolic extract with same concentration at 500µg could inhibit S. enterica Enteritidis with inhibition diameter of 9 and 7 mm., respectively.
Antibacterial Activity of C. asiatica against Bacillus species
The antibacterial activity of each extract against B. cereus was shown in table 1. Ethanolic and chloroform extract were not significant different in antibacterial activity against B. cereus with inhibition zone of 1.033± 0.052a and 0.983 ± 0.041ab cm., respectively. Hexane extract showed significant lower in antibacterial activity compare to ethanolic extract but not significant different from chloroform extract with inhibition zone of 0.941 ± 0.067b cm.
Pitinidhipat and Yasurin [7] found out that using 95% ethanolic extract of C. asiatica gave 1.67 ± 0.94 cm. inhibition zones against B. cereus. Also from the work of Sekar et al. [8], they found out that chloroform extract of C. asiatica could inhibit B. cereus with inhibition zone of 11 mm. at 50 µg/ml concentration.
From table 1, the antibacterial activity of each extract against B. subtilis was shown. The antibacterial activity of ethanolic extract was significant different from chloroform extract with inhibition zone of 1.050± 0.084a and 0.983 ± 0.041b cm., respectively. Hexane extract showed significant lower in antibacterial activity with inhibition zone of 0.875 ± 0.069c cm. Similar to our research, Sultan et al. [9] found out that ethanolic extract of C. asiatica could inhibit growth of B. subtilis with inhibition zone of 4 to 5 mm. at concentration of 400 µg. Another work from Jagtap et al. [10], showed that ethanolic extract of C. asiatica could inhibit growth of B. subtilis with inhibition zone of 10 mm. at concentration of 62.5 µg/ml. Perumal Samy and Ignacimuthu [11] found out that hexane extract of C. asiatica could inhibit growth of B. subtilis with inhibition zone of 10-15 mm.
From the results in this study, it could be conclude that among all three solvents, ethanol seems to be the most effective choice of extraction solvent in inhibiting growth of B. cereus and B. subtilis compare to chloroform and hexane. While antibacterial activity against three salmonella, chloroform showed highest activity compare to ethanolic and hexane extract. At 32 mg/ml concentration of all extract didn’t show bactericidal effect (MBC). While at 8 mg/ml concentration inhibit effect can be seen (MIC) against all microorganisms tested.
Antioxidant activity (Total phenolic content and FRAP)
Table 2: Total phenolic content and Ferric reducing antioxidant potential of C. asiatica extracts
|
Extracts |
Total phenolic content (µg GAE/mg) |
FRAP (mmol Fe2+/mg) |
|
Ethanol |
23.8020 ± 0.5241 a |
6.4008 ± 0.0393 a |
|
Chloroform |
22.1718 ± 0.1403 a |
3.4779 ± 0.6744 b |
|
Hexane |
7.9612 ± 1.6350 b |
1.7693 ± 0.1279 c |
Note: Different superscript within a column showed significant different at p < 0.05
Not only in the aspect of antibacterial activity had C. asiatica possessed but also in term of the ability to catch free radical compounds. From the works of Ikigai et al. [12] and Otake and et al. [13], suggested that the antimicrobial activity of plant in form of extract is most likely due to the combined effects of adsorption of polyphenols to bacterial membranes with membrane disruption and subsequent leakage of cellular contents. Herbs and spices also rich in phenolic compounds and besides exerting antimicrobial effect they may preserve the foods by reducing lipid oxidation as they are reported to have significant antioxidant activity [14].
From the work of Brinkhaus [15] found out that the chemical constituent inside C. asiatica oil are flavone derivatives, sesquiterpenes, triterpenic steroids, triterpenic acids and triterpenic acid sugar esters. This could contribute to total phenolic contents and ferric reducing potentials as can be seen in table 2. The ferric ion reducing activities of C. asiatica showed that ethanol extract possessed highest ferric reducing activity followed by chloroform and hexane extract. Similar results were found in total phenolic contents. The ethanolic extract had highest total phenolic content. Even though ethanolic extract showed slightly higher total phenolic contents than chloroform extract. However there is no significant different in term of statistic. While hexane extract had lowest total phenolic content. Thus the different between total phenolic compound result and ferric reducing antioxidant potential could be suggested that all phenolic compounds might not attribute or act as reducing agents.
From this study, by using ethanol as extraction solvents yielded highest amount of reducing ability in which significantly different from chloroform and hexane extracts. Another point to be suggested was that by using different solvent in extraction affect the total phenolic content and FRAP value.
Chemical profile of C. asiatica
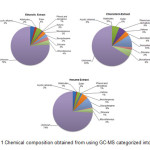 |
Figure 1: Chemical composition obtained from using GC-MS categorized into group Click here to View figure |
Different solvent systems have been used for the extraction of polyphenols from plant material [16]. Extraction yield is dependent on the solvent and the method of extraction [17]. Choosing solvents prove to be an important stepping stone in further development as it can be used as baseline for others. From this research, different solvents demonstrated different in antibacterial activity, antioxidant activity and chemical profile. In antibacterial activity, ethanolic and chloroform extract demonstrated slightly different in activity. Ethanolic extract showed higher antibacterial activity against B. cereus and B. subtilis than chloroform extract. On the other hand, antibacterial against three salmonella strains, chloroform showed highest activity. Hexane demonstrated lowest antibacterial activity among others. In antioxidant activity, there is no significant different in total phenolic content between ethanolic and chloroform extract. However hexane showed significant lower in total phenolic content than those two. In FRAP, all 3 solvents demonstrated significant different in FRAP value (Ethanol>Chloroform>Hexane).
Conclusion
The used of the medicinal plants or herbs was still based on traditional nostrum. In which in order to increase value of herbal products and medicinal plants, a knowledge-based scientific proved should be conducted. This research conducted tests on 3 different aspects of C. asiatica. There were antibacterial activity, antioxidant activity and chemical profiling. The result obtained showed that different extraction solvents affect all 3 aspects. For antibacterial, overall comparison showed no different between gram positive and gram negative bacteria. Ethanolic and chloroform extract showed slightly different in antibacterial activity. However hexane extract showed lowest antibacterial activity. In antioxidant part, ethanolic extract showed highest antioxidant activity (FRAP) and total phenolic content. In chemical profile, from identified peak, hexane extract showed widest variety of chemical compounds. Overall finding showed that C. asiatica would be a promising antibacterial and antioxidant activity source.
Methods
Preparation of Sample
C. asiatica was purchased from local markets in Bangkok, Thailand. The aerial part of C. asiatica was used. Fresh leaves were cleaned and trimmed into small pieces. Then, they were air dried in oven (Memmert UM500) at 45°C. The dried samples were finely ground into powder. The powders were kept at 4°C before used.
Preparation of Crude Extracts
C. asiatica powder was extracted with three different solvents; 95% v/v ethanol, hexane and chloroform using 1:10 ratio (g/ml). The mixtures were macerated at room temperature, 120 rpm, for 48 hours and then were filtered using whatman filter paper no.4. The crude extracts were concentrated using rotary evaporators at 45°C (BUCHI Rotavapor R-205) and then were diluted using dimethyl sulfoxide (DMSO), were kept at kept at -20°C before use.
Antibacterial activity Determination
The modified agar well diffusion method [18] was used in this experiment. The 100 µL of bacteria (approximately 1.5 × 108 CFU/ml) was swab on Mueller-Hinton agar (MHA) plate. The 100 µl of 50 mg/ml C. asiatica crude extracts were used to test antibacterial activity against S. enterica Typhimurium U302, S. enterica Enteritidis, S. enterica 4,5,12;i- human (US clone), B. cereus and B. subtilis using MHA plate. The 20 µl of 50 mg/ml penicillin G and DMSO was used as positive and negative control, respectively. The inhibition zones were measured to determine the effectiveness of the C. asiatica crude extract against each bacterium. The experiment was done in duplicate and three replications independently.
Minimum inhibitory concentration (MIC) and Minimum bactericidal concentration (MBC) Determination
The broth dilution method [19] was used for minimum inhibitory concentration (MIC) determination using Mueller-Hinton broth (MHB). The C. asiatica crude extracts’ concentrations were between 0 – 32 mg/ml. For Minimum bactericidal concentration (MBC) determination, the negative MIC tubes were streaked using MHA. The plates were incubated at 37°C for 24 hours. The experiment was done in duplicate and three replications independently.
Antioxidant activity test by Total phenolic content
The modified Folin–Ciocalteu method [20] was used for total phenolic content determination in C. asiatica crude extract. The 20 μl of 10 mg/ml C. asiatica crude extract was added to 1.58ml distilled water and 100μl Folin–Ciocalteu phenol reagent. The mixture was then allowed to stand for 8 minutes 30 seconds and 300μl sodium carbonate was added to the mixture and then this mixture was incubated at room temperature and without light for 30 minutes and then observed optical density (OD) at 765 nm. The results were expressed as microgram gallic acid equivalent (GAE/ml). The experiment was done in triplicate and three replications independently.
Antioxidant activity test by Ferric reducing antioxidant potential assay (FRAP)
The modified ferric reducing antioxidant potential assay [21] was used to determine FRAP value of C. asiatica crude extracts. The FRAP reagent was prepared using 300 mmol sodium acetate buffer at pH 3.6, 20 mmol iron chloride and 10 mmol 2,4,6-tripyridyl-s-triazine dissolved in 40 mmol hydrochloric acid at a ratio of 10:1:1 (v:v:v). The reagent was incubated at 37°C for 10 min before use. The 20μl of 1 mg/ml the extract was added, followed by adding 1000μl of FRAP reagent vigorously and kept in the dark for 30 min. The absorbance of this mixture was measured at 593 nm. FRAP values were expressed as mmol Fe2+/mg of sample. All measurements were done in triplicate and three replications independently.
Chemical Profile analysis
Component of crude extracts were analyzed by Gas Chromatography – Mass Spectrometry (GC-MS). The analysis was performed using Agilent Technologies 7890A GC System, 5975C inert XL EI/el MSD with triple-axis detector and GC sampler 80 using HP-5MS (30 m x 250 μm; 0.25 μm film thickness) column, using He as carrier gas, sample injection volume was 500 μl. The oven temperature was 35°C hold for 3.3 minutes, 90°C at 2.5°C/min, 130°C at 10°C/min and 270°C hold for 18 min at 6°C/min. The obtained peaks were matched with NIST database year 2011.
Statistical analysis
All experiments were conducted in three replications and statistical analysis was accomplished using ANOVA with Duncan’s multiple range tests (p < 0.05) by SAS software version 9.3.
Acknowledgements
The author would like to extend gratitude to Ms. Napapan Pongpoungphet, Science Instrument Center, Rangsit University, for her comments and suggestion for GC-MS operation and discussions. The authors gratefully acknowledge Mrs. Roungdao Klinjapo, lecturer from Department of Food Technology, Assumption University for total phenolic content part. Special thanks to Mr. Waruntorn Kaewkeeree, graduate students from Assumption University who help in statistical analysis.
References
- Zheng W, Wang SY. Antioxidant activity and phenolic compounds in selected herbs. J Agric Food Chem. 2001,49(11): 5165-70.
- ash BK, Faruquee HM, Biswas SK, Alam, MK, Sisir SM, Prodhan UK. Antibacterial and antifungal activities of several extracts of Centella asiatica L. against some human pathogenic microbes. Life Sci Med Res. 2011,2011,1-4.
- Ariffin F, Heong Chew S, Bhupinder K, Karim AA, Huda N. Antioxidant capacity and phenolic composition of fermented Centella asiatica herbal teas. J Sci Food Agric. 2011,91(15): 2731-9.
- Zhang M, Hettiarachchy SN, Horax R, Kannan A, Praisoody MDA, Muhundan A, Mallangi CR. Phytochemicals, antioxidant and antimicrobial activity of Hibiscus sabdariffa, Centella asiatica, Moringa oleifera and Murraya koenigii leaves. J Med Plants Res. 2011,5,6672-80.
- Mamtha B, Kavitha K, Srinivasan KK, Shivananda PG. An in vitro study of the effect of Centella asiatica [Indian pennywort] on enteric pathogens. Indian J Pharmacol. 2004,36(1): 41.
- Lee TK, Vairappan CS. Antioxidant, antibacterial and cytotoxic activities of essential oils and ethanol extracts of selected South East Asian herbs. J Med Plants Res. 2011,5(1),5284-90.
- Pitinidhipat N, Yasurin P. Antibacterial activity of Chrysanthemum indicum, Centella asiatica and Andrographis paniculata against Bacillus cereus and Listeria monocytogenes under osmotic stress. AU J T. 2012,15(4),239-45.
- Sekar T, Ayyanar M, Pillai YJK. Phytochemical screening and antibacterial activity of leaf and callus extracts of Centella asiatica. Bangladesh J Pharmacol. 2011, 6(1),55-60.
- Sultan RA, Mahmood SBZ, Azhar I, Ahmed SW, Mahmood ZA. Biological Activities Assessment of Centella asiatica (Linn.). J Herbs, Spices Med Plants. 2014,20(3),319-27.
- Jagtap NS, Khadabadi SS, Ghorpade DS, Banarase NB, Naphade SS. Antimicrobial and antifungal activity of Centella asiatica (L.) Urban, Umbeliferae. Res J Pharm Technol. 2009, 2(2),328-30.
- Samy RP, Ignacimuthu S. Antibacterial activity of some folklore medicinal plants used by tribals in Western Ghats of India. J Ethnopharmacol. 2000,69(1),63-71.
- Ikigai H, Nakae T, Hara Y, Shimamura T. Bactericidal catechins damage the lipid bilayer. Biochim Biophys Acta. 1993,1147(1),132-6.
- Otake S, Makimura M, Kuroki T, Nishihara Y, Hirasawa M. Anticaries effects of polyphenolic compounds from Japanese green tea. Caries Res. 1991,25(6),438-43.
- Yanishlieva NV, Marinova E, Pokorný J. Natural antioxidants from herbs and spices. Eur J Lipid Sci Technol. 2006,108(9), 776-93.
- Brinkhaus B, Lindner M, Schuppan D, Hahn EG. Chemical, pharmacological and clinical profile of the East Asian medical plant Centella aslatica. Phytomedicine. 2000,7(5),427-48.
- Pinelo M, Rubilar M, Sineiro J, Nunez MJ. Extraction of antioxidant phenolics from almond hulls (Prunus amygdalus) and pine sawdust (Pinus pinaster). Food Chem. 2004,85(2), 267-73.
- Goli AH, Barzegar M, Sahari MA. Antioxidant activity and total phenolic compounds of pistachio (Pistachia vera) hull extracts. Food Chem. 2005,92(3),521-5.
- Clark AM, El‐Feraly AS, Li WS. Antimicrobial activity of phenolic constituents of Magnolia grandiflora L. J pharma sci. 1981,70(8),951-2.
- Witebsky FG, MacLowry JD, French SS. Broth dilution minimum inhibitory concentrations: rationale for use of selected antimicrobial concentrations. J clin Microbiol. 1979,9(5),589-95.
- Ragazzi E, Veronese G. Quantitative analysis of phenolic compounds after thin-layer chromatographic separation. J Chromatogr A. 1973,77(2),369-75.
- Benzie IF, Strain JJ. [2] Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999,299,15-27.

This work is licensed under a Creative Commons Attribution 4.0 International License.









