Brøsted Acidic Ionic Liquid ([Bmim]Hso4) Promoted Cyclocodensation Reaction: Synthesis of 3,4,5-Substituted Furan-2(5h)-Ones
Sajjad Salahi, Malek Taher Maghsoodlou,* and Nourallah Hazeri
Department of Chemistry, Faculty of Science, University of Sistan and Baluchestan, P. O. Box 98135-674 Zahedan, Iran. Corresponding Author Email: mt_maghsoodlou@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/310424
Article Received on :
Article Accepted on :
Article Published : 21 Oct 2015
1-Butyl-3-methylimidazolium hydrogen sulfate [bmim]HSO4: an acidic ionic liquid was used as a catalyst for the synthesis of heterocyclic compounds 3,4,5-substituted furan-2(5H)-ones from aniline derivatives, dialkyl acetylenedicarboxylates and aromatic aldehydes at 75 °C. Solvent-free conditions, shorter reaction time, and good yields (71-94%) are some of advantages.
KEYWORDS:[bmim]HSO4; aldehydes; dialkylacetylenedicarboxylates; aniline; furanones
Download this article as:| Copy the following to cite this article: Salahi S, Maghsoodlou M. T, Hazeri N. Brøsted Acidic Ionic Liquid ([Bmim]Hso4) Promoted Cyclocodensation Reaction: Synthesis of 3,4,5-Substituted Furan-2(5h)-Ones. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Salahi S, Maghsoodlou M. T, Hazeri N. Brøsted Acidic Ionic Liquid ([Bmim]Hso4) Promoted Cyclocodensation Reaction: Synthesis of 3,4,5-Substituted Furan-2(5h)-Ones. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=12056 |
Introduction
Recently, science and technology are shifting emphasis toward environmentally friendly and green organic processes1. Several significant principles of green chemistry are omission of toxic solvents from chemical synthesis and avoidance of hazardous solvents and waste generation2. Ionic liquids are salts consisting of ions, which exist in a liquid state at ambient temperature 3. Ionic liquids have great properties not only as superseded non-toxic solvents but also as catalysts or reagents in organic reactions 4-7. Ionic liquids have the advantages like low vapour pressure, recyclability, thermal stability and the ability to dissolve many organic substrates8.
Singh et al. synthesized 1-butyl-3-methylimidazolium hydrogen sulfate ionic liquid ([bmim]HSO4) and reported its application for the synthesis of coumarins (Scheme 1) 9. Afterwards, [bmim]HSO4 as an acidic ionic liquid has been used as an efficient IL catalyst for many organic transformations 10-11.
![Scheme 1. Preparation of 1-butyl-3methylimidazolium hydrogen sulfate ([bmim]HSO4).](http://www.orientjchem.org/wp-content/uploads/2015/10/Vol31_No4_Bros_Sajj_Sch1-150x129.jpg) |
Scheme 1: Preparation of 1-butyl-3methylimidazolium hydrogen sulfate ([bmim]HSO4). Click here to View scheme |
Heterocyclic compounds are important because of their biological, chemical, and technical importance12. Heterocyclic compounds include many natural products, for example vitamins, hormones, antibiotics, alkaloids, pharmaceuticals, herbicides, and dyes 13.
2(5H)-Furanones are ring heterocyclic applied synthetic units in organic synthesis and are key structural elements that present in many biologically active natural products 14. This core is the key structure to induce a wide range of biological activities like antimicrobial 15, antifungal 16, anti-inflamatory 17, anticancer 18 and anti-viral HIV-1 19. Butenolide synthon is the structural module found in multitude bioactive natural products such as sarcophine 1 and rubrolide 2, which is separated from the colonial tunicate Ritterela rubra 20, 21, as well as in synthetic drug benfurodil hemisuccinate 3 (Figure 1).
Among the available methods for the construction of butenolides, the nucleophilic reaction between zwitterions of amine and dialkyl acetylene dicarboxylates with aldehyde is the most convenient 22.
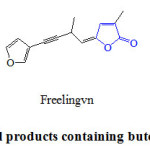 |
Figure 1: Natural products containing butenolide fragment. Click here to View figure |
Results and Discussion
In continuation of our efforts towards developing green catalysts for the synthesis of the heterocyclic compounds 23-25 and also in our efforts to continue produce furanones 26-29 we report herein, the synthesis of furanones from reaction between amines, dialkyl acetylene dicarboxylates and aldehyde in presence of ionic liquid [bmim]HSO4 (Scheme 2).
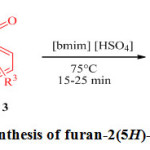 |
Scheme 2: Synthesis of furan-2(5H)-one derivatives. Click here to View scheme |
As a primary model for optimizing amount of catalyst loading, the reaction of benzaldehyde, aniline, and diethyl acetylenedicarboxylate was carried out in solvent free condition using different proportion of catalyst, Table 1. The best result obtained with 25% mol of catalyst. Higher percentage of catalyst decreases the yield of reaction.
| Table 1. Optimization amount of catalyst at 75°C for the synthesis of 3,4,5-substituted furan-2(5H)-ones in presence of ionic liquid [bmim] HSO4 | |||
|
Entry |
Catalyst (mol%) | Time (min) |
Isolated Yield |
|
1 |
15 |
20 |
——— |
|
2 |
15 |
40 |
46 |
|
3 |
20 |
20 |
78 |
|
4 |
25 |
20 |
93 |
|
5 |
30 |
20 |
84 |
Optimum result of temperature reaction is shown in table 2. In order to get the optimum results for the synthesis of 3,4,5-substituted furan-2(5H)-ones , the same reaction as above mentioned was done at different temperature. Maximum yield was achieved at 75°C in 20 minutes with 93 %.
| Table 2: Optimization of temperature for the synthesis of 3,4,5-substituted furan-2(5H)-ones in presence of ionic liquid [bmim] HSO4 | ||||
|
Entry |
Catalyst | Temperature (°C) | Time (min) |
Isolated Yield |
|
1 |
———– |
25 |
20 |
———- |
|
2 |
[bmim][HSO4] |
25 |
20 |
72 |
|
3 |
[bmim][HSO4] |
45 |
20 |
81 |
|
4 |
[bmim][HSO4] |
65 |
20 |
87 |
|
5 |
[bmim][HSO4] |
75 |
20 |
93 |
|
6 |
[bmim][HSO4] |
85 |
20 |
84 |
|
7 |
[bmim][HSO4] |
95 |
20 |
81 |
The suggested mechanism for the preparation of 4 is shown in scheme 3. Initially amine 1 adds on to dialkyl acetylenedicarboxylate 2 to produce the zwitterionic intermediate A which adds to activated aldehyde with the catalyst B to form C and the latter undergoes intra-molecular addition with concomitant alcohol elimination to give the lactone 4.
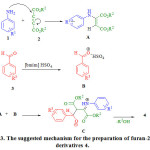 |
Scheme 3: The suggested mechanism for the preparation of furan-2(5H)-one derivatives 4. |
In summary, we have developed an efficient three-component reaction for the synthesis of 3,4,5-substituted furan-2(5H)-ones from reaction between anilines, dialkylacetylenedicarboxylates and aromatic aldehydes at 75 °C using ionic liquid [bmim]HSO4. The one-pot operational simplicity, good to high yields and easy work-up are the prominent advantages of this method.
Experimental
Chemistry
Melting points and IR spectra of all compounds were measured on an Electrothermal 9100 apparatus and a JASCO FTIR 460 Plus spectrometer, respectively. The 1H and 13C NMR spectra were recorded on a Bruker DRX-400 Avance instrument with CDCl3 as solvent at 400 and 100 MHz, respectively. Elemental analyses were performed using a Heraeus CHN-O-Rapid analyzer. The mass spectra were recorded on an Agilent Technology (HP) spectrometer operating at an ionization potential of 70 eV. All reagents were purchased from Merck (Darmastadt, Germany), or Fluka (Buchs, Switzerland), and used without further purification.
General Procedure
The mixture of aldehyde (1.0 mmol), amine (1.0 mmol), dialkylacetylenedicarboxylate (1.0 mmol) and acidic ionic liquid [bmim] HSO4 (25 mol %) were stirred at 75 °C. After completion of the reaction (monitored by thin-layer chromatography, TLC), the reaction mixture was washed with water, then, washed with ethanol (3×3 ml) to obtain a pure product. Spectral data of unknown products are represented below.
With the optimized conditions, the performance of the reaction was studied using a variety of anilines, aldehydes and dialkyl acetylenedicarboxylate to generate furan-2(5H)-one derivatives. The results are summarized in Table 3. These results indicate the effectiveness of electron-withdrawing and electron-releasing groups on the time and yield of the reaction. Benzaldehyde derivatives with electron-withdrawing groups react with aniline better than electron-donating groups for generation of furan-2(5H)-ones in good to high yields. In our research work, aliphatic aldehyde and aliphatic amine such as propanal and 1-buthylamin did not tolerate the reaction.
![3. Synthesis of furan-2(5H)-one derivatives in presence of ionic liquid [bmim] HSO4.](http://www.orientjchem.org/wp-content/uploads/2015/10/Vol31_No4_Bros_Sajj_T3-150x150.jpg) |
Table 3: Synthesis of furan-2(5H)-one derivatives in presence of ionic liquid [bmim] HSO4. Click here to View table |
Compound 4l
Colorless solid; tert-butyl 2,5-dihydro-5-oxo-4-(phenylamino)-2-p-tolylfuran-3-carboxylate 0.268 g (83%); mp 175-178 °C; IR (KBr): 3400, 3020, 2925, 1701, 1670 cm–1.1H NMR (400 MHz, CDCl3) δ: 1.38 (s, 9H, CH3), 2.28 (s, 3H, CH3), 5.64 (s, 1H, Hbenzylic), 7.04-7.50 (m, 9H, HAr), 9.29 (br, 1H, NH); 13C NMR (100 MHz, CDCl3) δ: 165.1 and 162.9 (ester C=O), 156.7, 138.1, 136.4, 132.1, 129.1, 128.9, 127.4, 125.6, 122.3 and 114.5 (CAr), 83.2 (C-O), 61.4 (C benzylic), 28.0 (3 CH3), 21.1 (CH3). MS m/z (%): 57 (44), 77 (33), 91 (27), 115 (58), 144 (99), 189 (100), 264 (61), 291 (19), 309 (94), 365 (M+, 50). Anal. calcd. for C22H23NO4: C, 72.31; H, 6.34; N, 3.83. Found: C, 72.49; H, 6.40; N, 3.87.
Compound 4m
Colorless solid; tert-butyl 2-(4-chlorophenyl)-2,5-dihydro-5-oxo-4-(phenylamino)furan-3-carboxylate mp 169-170 °C; IR (KBr): 3265, 3060, 2925, 1710, 1672 cm–1; 1H NMR (400 MHz, CDCl3) δ: 1.39 (s, 9H, CH3) 5.67 (s, 1H, Hbenzylic), 7.11-7.46 (m, 9H, HAr), 9.32 (br, 1H, NH); 13C NMR (100 MHz, CDCl3) δ: 164.8 and 162.7 (ester C=O), 156.9, 136.0, 134.2, 134.0, 129.0, 128.9, 128.7, 126.0, 122.4, and 114.1 (10 CAr and Cvinyl), 83.5 (C-O), 60.9 (Cbenzylic), 28.0 (CH3). MS m/z (%): 57 (100), 77 (63), 164 (83), 209 (41), 284 (35), 329 (71), 385 (M+,18), 387 (M+ +2, 6), 389 (M+ +4, 0.3). Anal. calcd. for C21H20ClNO4 : C, 65.37; H, 5.22; N, 3.63. Found: C, 65.52; H, 5.30; N, 3.69.
Acknowledgements
We gratefully acknowledge financial support from the Research Council of the University of Sistan and Baluchestan.
References
- Zhang , w and Berkeley, w. c. Green Techniques for Organic Synthesis and Medicinal Chemistry Wiley, Chichester (2012).
- Yavari, I and Kowsari, E. Tetrahedron Lett. 2007, 48, 3753.
- Zhao, D.; Wu, M .; Kou, Y.; Min, E. Catalysis Today. 2002, 74, 157.
- Wasserscheid, P and Keim, W. Angew. Chem. Int. Ed. 2000, 39, 3772.
- Dupont, J.; Souza, R. F .; Suarez, P. A. Z. Chem. Rev. 2002, 102, 3667.
- Wilkes, J. S. Green Chem. 2002, 4, 73.
- Chrobok, A.; Baj, S.; Pudło, W.; Jarzebski, A. Appl. Catal. A: Gen. 2009, 366, 22.
- Kwan, J.; Kim, M. J. J. Org. Chem. 2002, 67, 6845.
- Singh, V.; Kaur, S.; Sapehiyia, V.; Singh, J.; Kad, G. L. Catal. Commun. 2005, 6, 57.
- Gupta, N.; Sonu Kad. G. L.; Singh, J. Catal. Commun. 2007, 8, 1323.
- Tajik, H.; Niknam, K.; Parsa, F. J. Iran. Chem. Soc. 2009, 6, 159.
- Jouler, J. A.; Mills, K. Heterocyclic chemistry. Wiley, United Kingdom(2010).
- H. Veisi and R. Ghorbani-Vaghei, Tetrahedron. 2010, 66, 7445.
- Rao, Y. S.; Chem. Rev. 1976, 76, 625.
- (a) Rossi, R.; Bellina, F.; Biagetti,M .; Mannina, L. Tetrahedron Lett. 1998, 39, 7799.; (b) Grossmann, G.; Poncioni, M.; Bornand, M.; Jolivet, B.; Neuburger. M.; Sequin, U. Tetrahedron. 2003, 59, 3237. (c) Levy, L. M.; Cabrera, G. M.; Wright, J. E.; Seldes, A. M. Phytochemistry. 2003, 62, 239.
- (a) Hein, S. M.; Gloer, J. B.; Koster, B.; Malloch, D. J. Nat. Prod. 2001, 64, 809 .; (b) Pour, M.; Spulak, M.; Balsanek, V.; Kunes, J.; Kubanova, P.; Butcha, V. Med. Chem. 2003, 11, 2843.
- Padakanti, S.; Pal, M.; Yeleswarapu, K. R. Tetrahedron. 2003, 59, 7915.
- (a) Hoye, T. R.; Tan, L. Tetrahedron Lett. 1995, 36, 1981. (b) Takahashi, S.; Kubota, A.; Nakata, T. Tetrahedron Lett. 2002, 43, 8661; (c) Bellina, F.; Falchi, E.; Rossi, R. Tetrahedron, 2003, 9091, 59.
- (a) Hanessian, S.; Park, R. Y.; Yang, R. Y. Synlett. 1997, 35. (b) Hanessian, S.; Park, R. Y.; Yang, R. Y. Synlett. 1997, 353. (c) Choudhury, A.; Jin, F.; Wang, D.; Wang, Z.; Xu, G.; Nguyen, D.; Castoro, J.; Pierce, M. E.; Confalone, P. N. Tetrahedron Lett. 2003, 44, 247.
- Miao, S.; Andersen, R. J. J. Org. Chem. 1991, 56, 6275 .
- Kotora, M.; Negishi, E. Synthesis. 1997, 121.
- Narayana, S.; Murthy, B.; Madhav, A.; Vijay Kumar, K.; Rama ,R.; Nageswar, Y. V. D. Tetrahedron. 2009, 65, 5251.
- Hazeri, N.; Sajadikhah, S. S.; Maghsoodlou, M. T.; Norouzi, M.; Moein, M.; Mohamadian-Souri, S. J. J. Chem. Res. 2013, 550 .
- Mousavi, M. R.; Hazeri, N.; Maghsoodlou, M. T.; Salahi, S.; Habibi-Khorassani, S. M. Chin. Chem. Lett. 2013, 24, 411.
- Hazeri, N.; Maghsoodlou, M. T.; Mahmoudabadi, N.; Doostmohammadi, R.; Salahi, S. Current Organocatal. 2014, 1, 45.
- Doostmohammadi, R.; Maghsoodlou, M. T.; Hazeri, N.; Habibi- Khorassani, S. M. Res. Chem. Intermed. 2013, 39, 4061.
- Doostmohammadi, R.; Hazeri, N. Lett. Org. Chem. 2013, 10, 199.
- Doostmohammadi, R.; Maghsoodlou, M. T.; Hazeri, N.; Habibi-Khorassani, S. M. Chin. Chem. Lett. 2013, 24, 901.
- Maghsoodlou, M. T.; Habibi-Khorassani, S. M.; Moradi, A.; Hazeri, N.; Davodi, A.; Sajadikhah, S. S. Tetrahedron. 2011. 67, 8492.

This work is licensed under a Creative Commons Attribution 4.0 International License.









