Quantum Mechanical Study on the Adsorption of Drug Gentamicin onto γ-Fe2O3 nanoparticles
A. Mansoorinasab, A. Morsali *, M. M. Heravi, and S. A. Beyramabadi
Department of Chemistry, Mashhad Branch, Islamic Azad University, Mashhad, Iran and Research Center for Animal Development Applied Biology, Mashhad Branch, Islamic Azad University, Mashhad 917568, Iran. Correspondence author E-mail: almorsali@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/310329
Article Received on :
Article Accepted on :
Article Published : 31 Aug 2015
In this work, using quantum mechanics, the interaction of drug gentamicin with γ-Fe2O3 nanoparticles have been studied. Fe2O3 nanoparticles were modeled using Fe6(OH)18(H2O)6 ring clusters. gentamicin molecule can coordinate to the γ-Fe2O3 nanoparticles via its own OH or NH2 groups. All of the calculations have been performed using a hybrid density functional method (B3LYP) in solution phase. Three possible modes of noncovalent interaction of gentamicin onto γ-Fe2O3 nanoparticles were investigated. Quantum molecular descriptors in the drug-nanoparticle systems were studied. It was found that binding of gentamicin with γ-Fe2O3 nanoparticles is thermodynamically favorable. Among NCOOH and NCOCl, the first one has more binding energy and can act as a suitable system for drug gentamicin delivery within biological systems (noncovalent). Gentamicin can intract with γ-Fe2O3 nanoparticles via OH and NH2 groups which the first one has more binding energy.
KEYWORDS:Gentamicin; γ-Fe2O3 nanoparticles; DFT; Drug delivery; Quantum molecular descriptors
Download this article as:| Copy the following to cite this article: Mansoorinasab A, Morsali A, Heravi M. M, Beyramabadi S. A. Quantum Mechanical Study on the Adsorption of Drug Gentamicin onto γ-Fe2O3 nanoparticles. Orient J Chem 2015;31(3). |
| Copy the following to cite this URL: Mansoorinasab A, Morsali A, Heravi M. M, Beyramabadi S. A. Quantum Mechanical Study on the Adsorption of Drug Gentamicin onto γ-Fe2O3 nanoparticles. Orient J Chem 2015;31(3). Available from: http://www.orientjchem.org/?p=10515 |
Introduction
Gentamicin is a commonly used antibiotic which prevents bacterial infection around the implant. It is an aminoglycoside antibiotic, and can treat many types of bacterial infections, particularly Gram-negative infection. It is also one of the few heat-stable antibiotics that remains active even after autoclaving, and this makes it particularly useful in the preparation of certain microbiological growth media1.
Regarding the increasing use of nanotechnology in today’s life, understanding of the functional mechanism of nanoparticles is of great importance. The rapid development of nanoscience has opened new ways in timely and prompt diagnosing of diseases and drug delivery. The use of nanoparticles in drug delivery is a new field which is rapidly developing2-5.
Jayarathne et al. presented a model for γ-Fe2O3 nanoparticles on the basis of Fe6(OH)18(H2O)6 ring cluster which is in good consistency with the experimental data including vibration frequencies and bond lengths6. The huge surface, easy separation and low cost are the reasons to use γ-Fe2O3 nanoparticles as the strong adsorbent materials.
In spite of extensive use of magnetic nanoparticles, so far, molecular mechanism of adsorption of drugs in water by these nanoparticles has not been investigated. In this work, using quantum mechanical methods, the noncovalent adsorption of gentamicin onto γ-Fe2O3 nanoparticles was studied.
Computational Details
All of the present calculations have been performed with the B3LYP7-9 hybrid density functional level using the GAUSSIAN 03 package10. The 6-31G(d,p) basis sets were employed except for Fe where the LANL2DZ basis set was used with effective core potential (ECP) functions.
The solvent has an important role in chemical reactions explicitly or implicitly11-15. The implicit effects of the solvent was considered by using the polarized continuum model (PCM)16. In the PCM method, the molecular cavity is made up of the union of interlocking atomic spheres. All degrees of freedom for all geometries were optimized in solution (water).
Results and Disscussion
The properties of γ-Fe2O3 nanoparticles were modeled using Fe6(OH)18(H2O)6 ring clusters of six edge sharing octahedra joining via 12 OH groups. The 6 water molecules and 6 surface OH groups were expected to form a network of H-bonded interactions (Fig. 1). The optimized geometries of gentamicin (GEN), Fe6(OH)18(H2O)6 or γ-Fe2O3 nanoparticle (NP) in solution phase are shown in Fig. 1. Gentamicin is a non-planar molecule with the amino and hydroxyl groups protruding out of the molecular plane as shown in Fig. 1.
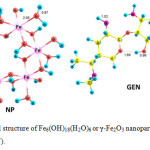 |
Figure1: Optimized structure of Fe6(OH)18(H2O)6 or γ-Fe2O3 nanoparticle (NP) and gentamicin (GEN). Click here to View figure |
Three possible modes of noncovalent interaction of Gentamicin onto γ-Fe2O3 nanoparticles were studied. Figures 2-4 present these configurations in solution phase, namely, GEN/NP1-GEN/NP3. Gentamicin may interact with γ-Fe2O3 nanoparticles through amino ( GEN/NP1 ) and hyroxyl (GEN/NP2) groups to form hydrogen bonds. In GEN/NP3 parallel orientation was considered.
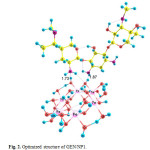 |
Figure 2: Optimized structure of GEN/NP1. Click here to View figure |
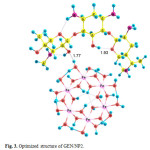 |
Figure 3: Optimized structure of GEN/NP2. Click here to View figure |
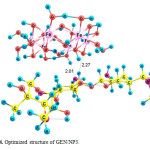 |
Figure 4: Optimized structure of GEN/NP3. Click here to View figure |
The binding energies (Eb) of gentamicin whit γ-Fe2O3 nanoparticles are calculated using the following equation and presented in Table 1:
Eb = EGEN|NP – (ENP– EGEN) (1)
Using the calculated binding energies of GEN/NP1-GEN/NP3 in Table 1, these energies are negative in solution phase indicating gentamicin is stabilized by γ-Fe2O3 nanoparticles surface. Among the 3 configurations in the solution phase, the configuration 2, in which the drug interacts with γ-Fe2O3 nanoparticles through hyroxyl (GEN/NP2) group, is the most stable configuration.
In describing stability and chemical reactivity of different systems, quantum molecular descriptors have been used like the chemical potential, the global hardness, the electrophilicity index and etc.
The chemical potential (μ) which shows escape tendency of an electron from equilibrium, is defined as follows:
where is the ionization potential and is the electron affinity of the molecule.
The global hardness (μ) shows the resistance of one chemical species against the change in its electronic structure (Equation (3)). Increase η in causes an increase in the stability and a decrease in reactivity.
η = (I-A)/2 (3)
Electrophilicity index (ω) was defined by Parr as follows17:
ω = μ2 |2η (4)
Tabels 1 represents the values of the quantum molecular descriptors calculated for GEN, NP and GEN/NP1-3 in solution phases. In this table, besides quantum molecular descriptors, Eg (HOMO-LUMO energy gap) was also presented. Eg notably shows a more stable system.
Table 1: Bonding energies (kJ/mol) quantum molecular descriptors (eV) for optimized geometries.
|
Species |
Eb |
EHOMO |
ELUMO |
Eg |
I |
A |
|||
|
GEN |
– |
-5.95 |
-1.85 |
4.11 |
5.95 |
1.85 |
-3.90 |
2.05 |
3.70 |
|
NP |
– |
-5.58 |
-4.48 |
1.10 |
5.58 |
4.48 |
-5.03 |
0.55 |
22.95 |
|
GEN/NP1 |
-74.61 |
-5.93 |
-4.33 |
1.60 |
5.93 |
4.33 |
-5.13 |
0.80 |
16.39 |
|
GEN/NP2 |
-94.35 |
-6.09 |
-3.30 |
2.79 |
6.09 |
3.30 |
-4.69 |
1.40 |
7.88 |
|
GEN/NP3 |
-86.82 |
-5.94 |
-3.27 |
2.67 |
5.94 |
3.27 |
-4.60 |
1.33 |
7.94 |
According to the data in Table 1, , I, and Eg related to the gentamicin drug are higher than GEN/NP1-3, showing the stability of the gentamicin decreases in the presence of γ-Fe2O3 nanoparticles and its reactivity increases. Also, in confirmation of the previous issue, it is observed that of the gentamicin becomes more negative in the presence of γ-Fe2O3 nanoparticles. of the gentamicin increases in the presence of γ-Fe2O3 nanoparticles, showing that the gentamicin acts as electron acceptor.
Six H2O molecules, due to weaker bond relative to OH, are proper choices which leave the cluster and are replaced by gentamicin. Scheme 1 shows the mechanism of covalent adsorption of GEN onto γ-Fe2O3 nanoparticles where and are equilibrium constants and is rate constant (using GEN/NP2 as reactant).
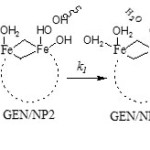 |
Scheme 1 Click here to View scheme |
species GEN/NP2 is initially formed with equilibrium constant K1 , following which the species GEN/NPC and product are formed in k1 and K´1 paths. The optimized structures of GEN/NPC has been shown in Fig. 5 (covalent adsorption). GEN/NPC is more stable than GEN/NP2 by 49.31 kJ/mol, so GEN/NPC is thermodynamic product.
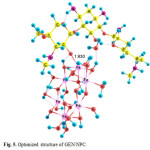 |
Figure 5: Optimized structure of GEN/NPC. Click here to View figure |
Conclusion
Using density functional theory, the effects of the adsorption of gentamicin onto γ-Fe2O3 nanoparticles have been studied in detail in solvent environment. Three possible modes of noncovalent interaction of Gentamicin onto γ-Fe2O3 nanoparticles were investigated. Besides parallel interaction (GEN/NP3), there are two possibilities for the formation of hydrogen bonds between gentamicin and γ-Fe2O3 nanoparticles, for the first possibility, gentamicin is interacted with γ-Fe2O3 nanoparticles through amino groups (GEN/NP1) and for the second one through hydroxyl groups (GEN/NP2). The product of second possibility is more stable.
Acknowledgment
We thank the theoretical research group in research center for animal development applied biology for allocation of computer time.
References
- Popat, K. C.; Eltgroth, M.; LaTempa, T. J.; Grimes, C. A.; Desai, T. A. Biomaterials 2007, 28, 4880-4888.
- Sun, C.; Lee, J. S.; Zhang, M. Adv. drug deliver. rev. 2008, 60, 1252-1265.
- Hosseini, F.; Seyedsadjadi, M.; Farhadyar, N. Orient. J. Chem. 2014, 30, 1609-1618.
- Veiseh, O.; Gunn, J. W.; Zhang, M. Adv. drug deliver. rev. 2010, 62, 284-304.
- Yallapu, M. M.; Othman, S. F.; Curtis, E. T.; Gupta, B. K.; Jaggi, M.; Chauhan, S. C. Biomaterials 2011, 32, 1890-1905.
- Jayarathne, L.; Ng, W.; Bandara, A.; Vitanage, M.; Dissanayake, C.; Weerasooriya, R. Colloid surface A 2012, 403, 96-102.
- Becke, A. D. J. Chem. Phys. 1993, 98, 5648-5652.
- Becke, A. D. Phys. Rev. A 1988, 38, 3098-3104.
- Lee, C.; Yang, W.; Parr, R. G. Phys. Rev. B 1988, 37, 785-790.
- Frisch, M.; Trucks, G.; Schlegel, H.;Scuseria, G.; Robb, M.;Cheeseman, J.; Montgomery, J.;Vreven, T.;Kudin, K.;Burant, J. Gaussian 03, Revision B.05Gaussian Inc., Pittsburgh, PA,2003.
- Akbari, A.; Hoseinzade, F.; Morsali, A.; Ali Beyramabadi, S. Inorg. Chim. Acta 2013, 394, 423-429.
- Morsali, A.; Hoseinzade, F.; Akbari, A.; Beyramabadi, S. A.; Ghiasi, R. J. Solution Chem. 2013, 42, 1902-1911.
- Magham, A. H. J.; Morsali, A.; Beyramabadi, S.; Chegini, H. Progress in Prog. React. Kin. Mec. 2015, 40, 119-127.
- Gharib, A.; Morsali, A.; Beyramabadi, S.; Chegini, H.; Ardabili, M. N. Prog. React. Kin. Mec. 2014, 39, 354-364.
- Darzi, N.; Morsali, A.; Beyramabadi, S. A. Orient. J. Chem. 2015, 31, 1121-1125.
- Tomasi, J.; Persico, M. Chem. Rev. 1994, 94, 2027-2094.
- Parr, R. G.; Szentpaly, L. v.; Liu, S. J. Am. Chem. Soc. 1999, 121, 1922-1924.

This work is licensed under a Creative Commons Attribution 4.0 International License.









