Novel Polymer Nanocomposite With Silicon Carbide Nanoparticles
Alyona I. Wozniak, Vitaly S. Ivanov, Vasily M. Retivov, Anton S. Yegorov*
Federal State Unitary Enterprise «State Scientific Research Institute of Chemical Reagents and High Purity Chemical Substances» (FSUE «IREA») 3,Bogorodsky val, Moscow, 107076, Russia. Corresponding Author.E-mail:egorov@irea.org.ru
DOI : http://dx.doi.org/10.13005/ojc/310332
Article Received on :
Article Accepted on :
Article Published : 21 Aug 2015
Polyimides are ranked among the most heat-resistant polymers and are widely used in high temperature plastics, adhesives, dielectrics, photoresistors, nonlinear optical materials, membrane materials for gasseparation, and Langmuir–Blodgett (LB) films, among others. While there is a variety of high temperature stable polyimides, there is a growing demand for utilizing these materials at higher temperatures in oxidizing and aggressive environments. Therefore, we sought to use oxidation-resistant materials to enhance properties of the polyimide composition maintaining polyimide weights and processing advantages. In this paper we introduced results of utilizing inorganic nanostructured silicon carbide particles to produce an inorganic particle filled polyimide materials.
KEYWORDS:particle-reinforced nanocomposite; silicon carbide; polyimide
Download this article as:| Copy the following to cite this article: Wozniak A. I, Ivanov V. S, Retivov V. M, Yegorov A. S. Novel Polymer Nanocomposite With Silicon Carbide Nanoparticles. Orient J Chem 2015;31(3). |
| Copy the following to cite this URL: Wozniak A. I, Ivanov V. S, Retivov V. M, Yegorov A. S. Novel Polymer Nanocomposite With Silicon Carbide Nanoparticles. Orient J Chem 2015;31(3). Available from: http://www.orientjchem.org/?p=10395 |
Introduction
In order to maintain further rapid development of aircraft industry, rocket construction, astronautics, nuclear power production, electronics industry, radio engineering and other fields of industry, the polymer-based materials with high durability, thermal stability, elasticity and resistance to ionizingradiation are needed. As a result research efforts in this direction, a new class of polymers with cyclic chain-polyimides was discovered[1].
Recent research trends have been focused on attempts to combine polymer structures and nanoparticles in order to produce materials with high stiffness, toughness and tribological properties [2]. This approach is based on the recently published evidence of ability to improve and modify material properties by adding bulk nano fillers to the polymer matrix core[3-7].
Aeronautics is being viewed as one of the most prospective fields in terms of composite materials consumption. In aircraft industry metal or ceramic structures are getting widely replaced with fiber reinforced polymer composites. Introducing composite materials into aircraft technology caused dramatic improvement of work characteristics.One of the most important advantages observed was significant weight reduction (up to 60-80% lighter compared to aluminum) which helps to cut fuel consumption and operation costs. Other benefits of using composites include avoiding corrosion issues and ability to produce complex shapes which are hard to achieve by use of ceramic materials. Since metal alloys and aluminum have well known functional limitations and high costs, the steady growth of composite demand is expected, while the search for new affordable composite solutions with better working properties remains extremely important. In order to be considered as equal or advantageous functional substitution of metal and ceramics, these new materials have to demonstrate excellent stability under extreme conditions of flame and intense.
One of the recent examples of aircraft composite technology success would be the F-35 Lightning II by Lockheed Martin, which will be the first mass-produced aircraft to feature structural nanocomposites in non-load bearing air frame components. The material being used is identified as “advanced polymers engineered for the extreme- first generation”, or APEX. It is described within the display as “best-in-class ultra-lightweight and affordable structural thermoplastic enhanced with nanoparticles that delivers increased mechanical properties, thermal stability, electrical conductivity and process ability over currently available projects” [8].
In current project silicon carbide (SiC) nanoparticles were preferred over other nano species due to their unique physical properties such as excellent chemical resistance, heat resistance, high electron mobility, excellent thermal conductivity and outstanding mechanical properties. They are known to be used for high-performance composites [9-12] and in electronics [13, 14]. These properties make SiC nanoparticles a suitable material for the production of polymer nanocomposites with reinforced structure [10]. However, there is only one mention of polyimide/nano-SiC composite. Bazzar et al. prepared poly(triazole-imide)/SiC nanocomposites using a polymerizationmethod [15]. The poly(amic acid)/SiC nanocomposites were prepared via the reaction between diamine and dianhydride in the presence of SiC nanoparticles, and then under went thermal heating in vacuum to form poly(triazole-imide)/SiC nanocomposites. In comparison with poly(triazole-imide), the poly(triazole-imide)/SiC nanocomposites had the tensile strength increase from 108 to 165 MPa and exhibited high thermal stability (5% weight loss with the temperature increasing from 380 to 500°C).
Our initial work was focused on studying the effects of nanostructured SiC (at loading level of 6 wt%) on enhancement of the thermal durability of the polyimides. In this work we used poly-oxydiphenylene-pyromellitimide (see Fig. 1) which is one of the most widely used polyimides in civil and military aircraft production.
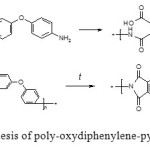 |
Figure1: Synthesis of poly-oxydiphenylene-pyromellitimide |
Experimental section
Materials
1-Methyl-2-pyrrolidinone (NMP, Component-Reaktiv) was purified prior to use in poly(amic acid) synthesis by drying over calcium hydride for at least 8 hours, followed by distillation under reduced pressure (using a vacuum pump) (b.p. 202ºC/760mmHg).
4,4’-Oxydianiline (4,4’-ODA, Vekton) was sublimed under vacuum (~5 torr) at ~155ºC.
Pyromellitic dianhydride (PMDA) was synthesized according to the literature [16].
Silicon carbide (SiC) nanoparticles were purchased from InKhimSintez. The properties of the SiC-nanopowder are listed in Table 1.
Table 1: Characteristic properties of the SiC nanoparticles
Purity |
97 % |
Averageparticlediameter |
70-80 nm |
Surfacearea |
30 – 35 m2/g |
Colour |
Black |
Morphology |
Nearlyspherical |
Bulkdensity |
<0.5 g/cm3 |
Preparation of the Nanocomposite
0.6153 g of nanostructured SiC and NMP (50 mL) were sonicated into a three-necked round-bottom flask equipped with a condenser and an argon inlet for 15 min. Then 5.0461 g (25.2 mmol) of ODA was added and sonication continued for another 15 min, afterwards 5.5000 g (25.2 mmol) of PMDA was added portion wise. The mixture was sonicated for 30 min more. The resulting highly viscous poly(amic acid) solution was cast onto clean and dry glass plates by a scalpel; the films were dried in an vacuum oven at 80°C for 6 h and then at 150, 200, 250, and 300 °C for 1 h at each temperature followed by final drying step at 350 °C for 15min.
Analytical Techniques
Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) analysis was collected with a TA Instruments SDT Q600, under atmosphere of argon and air at a heating rate of 10ºC/min from room temperature to 800ºC.
For scanning electron microscopy (SEM), a Hitachi SU-1510 VP-SEM was used under accelerating voltage of 7 kV and a working distance of 5.5mm.IR-spectra were recorded with a VERTEX 70 FT-IR, Bruker Optics.
Results and Discussion
Synthesis of Nanocomposite
The nano-SiC was mixed with 4,4’-ODA and PMDA under sonication to give the corresponding poly(amic acid) with uniformly distributed particles with viscosity of 4929,89 Pa·s. To achieve imidization,a common thermal imidization approach was used at the final stage of the reaction. The reaction mixture was cast on a clean glass plate, and the film was heated through various stages up to 350°C in vacuum to remove solvent and water formed by the imidization. Black opaque solid film was obtained.
Nanocomposite Properties
The transformation of poly(amic acid) intopolyimidewas confirmed by Fourier transform infrared (FTIR) spectroscopy(see Fig.2andFig. 3). FTIR spectra of the film prepared by the thermal imidization method (Fig. 3) showed absorption bands at about 1775 cm-1 (C=O asymmetric stretching), 1715 cm-1 (C=O symmetric stretching), 1368 cm-1 (C-N stretching), and 721 cm-1 (C=O bending), which are characteristic for imide rings. The absence of absorption bands at 1660 cm-1 corresponding to C=O amide stretching in the FTIR spectra of polyimides indicates complete imidization. Due to the low loading of nanofiller FTIR signals of SiC can’t be observed. FTIR spectra of the SiC particles are shown in Fig. 4.
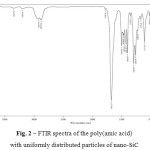 |
Figure 2: FTIR spectra of the poly(amic acid) with uniformly distributed particles of nano-SiC Click here to View figure |
 |
Figure 3: FTIR spectra of the nanocomposite film prepared by the thermal imidization method Click here to View figure |
 |
Figure 4: FTIR spectra of the SiC-nanoparticles Click here to View figure |
The thermal properties of the polyimide nanocomposite were evaluated by thermogravimetric analysis (TGA). The 5% weight loss temperature of this material is 593°C in air (compared with 530ºC of poly-oxydiphenylene-pyromellitimide without SiC nanopowder). TheTg of the nanocomposite was obtained from DSC measurements and found to be 460ºC. TGA/DSC curves of polyimide without nanofiller and nanocomposite film in air are shown in Fig. 5 and Fig. 6.
 |
Figure 5: TGA/DSC curve of poly-oxydiphenylene-pyromellitimidefilm in air Click here to View figure |
 |
Figure 6: TGA/DSC curve of nanocomposite film in air Solid film sample was examined by Scanning Electron Microscopy (SEM) to determine how the particles were dispersed in the polyimide (see Fig. 7). Click here to View figure |
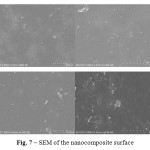 |
Figure 7: SEM of the nanocomposite surface Click here to View figure |
Conclusion
A new material based on polyimide and nanostructured SiC has been successfully synthesized and exhibited good thermal stability. Based on the results collected to date, the use of silicon carbide loadings represents significant interest for novel materials development. In short time periods and with little additional effort, the use of these particles improves thermal stability in comparison to the pure core polymer. Further studies are needed for detailed interpretation and understanding of current results. The future work on this project will be focused on producing and studying composite materials with various polyimides and different loadings of silicon carbide nanoparticles.
Acknowledgements
Applied researches are carried out with state financial support represented by the Ministry of Education of Russia under the Agreement on granting subsidies No.14.625.21.0003 of August 25, 2014. Unique identifier for Applied Scientific Researches (project) RFMEFI62514X0003.
References
- Bessonov, M. I.; Koton, M. M.; Kudryavtsev, V. V.; Laius, L. A. Polyimides: Thermally Stable Polymers; Plenum, New York. (1987)
- Zou, H.; Wu, S.; Shen, J. Chem. Rev. 2008, 108, 3893-3957
- Wang, P. J.; Lin, C. H.; Chang, S. L.; Shih, S. J. Polym. Chem. 2012, 3, 2867-2874
- Agag, T.; Koga, T.; Takeichi, T. Polymer2001, 42, 3399-3408
- Udmale, V.; Mishra, D.; Gadhave, R.; Pinjare, D.; Yamgar, R. Orient. J. Chem.2013, 29, 927-936
- Kherroub, D. E.;Belbachir, M.;Lamouri, S.;Bouhadjar, L.;Chikh, K. Orient. J. Chem.2013, 29, 1429-1436
- Sabzevari, O.;Marjani, A.;Daripour, A. Orient. J. Chem.2015, 31, 1091-1098
- Trimble, S. Flight International2011, 31/V-6/VI, 21-22
- Mavinakuli, P.; Wei, S.; Wang, Q.; Karki, A. B.; Dhage, S.; Wang, Z. J. Phys. Chem. Part C. 2010, 114, 3874-3882
- Guo, Z.; Kim, T. K.; Lei, K.; Pereira, T.; Sugar, J. G.; Hahn, H. T. Compos. Sci. Technol.2008, 68, 164-170
- Majewski, P.; Choudhury, N. R.; Spori, D.; Wohlfahrt, E.; Wohlschloegel, M. Mater. Sci. Eng. Part A.2006, 434, 360-364
- Bazzar,M.;Ghaemy,M.;Alizadeh,R.Polym. Deg. Stab.2012, 97, 1690-1703
- Rittenhouse, T. L.; Bohn, P. W.; Hossain, T. K.;Adesida, I.; Lindesay, J.; Marcus, A.J. Appl. Phys.2004, 95, 490-496
- Fan, J. Y.; Wu, X. L.; Kong, F.; Qiu, T.; Huang, S. G. Appl. Phys. Lett.2005, 86, 171903, 1-3
- Bazzar, M.;Ghaemy, M. Compos. Sci. Technol.2013,86, 101−108
- Richter, G. A.Veba-Chemie A.G,US Patent 4014755, 1977.

This work is licensed under a Creative Commons Attribution 4.0 International License.









