Liquid-Liquid Extraction of Zinc By 3-Methyl-Quinoxaline-2-Thione From Nitrate Medium
Sanae Baki Senhaji*, Ahmed Elyahyaoui
Départ. de Chimie, Laboratoire de Radiochimie, Faculté des Sciences. B.P 1014, Rabat, Morocco. Corresponding Author E-mail : b.senhaji.sanae@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/310339
Article Received on :
Article Accepted on :
Article Published : 16 Sep 2015
The extraction of zinc with 3-methyl-2 (1H)-quinoxalinethione LH, is investigated in nitrate solution as a function of pHrange 2-6, and analytical concentrations of NO-3 anions (CNO3) and extractant (CLH). The extraction mechanism with LH,is determined on the basis of the variation of the distribution coefficient D with pH, CNO3 and CLH. As a result, the partition equilibrium involves complexing phenomenon, ion-pair formation, and 1H+ exchange reaction. The overall extraction constants are logK01.5 = 5.1, and logK11.5 = -0.6. The extraction complexes are found to be Zn(LH2)∫(NO3)∫+2 and Zn(L) (LH)∫-1 (NO3) with l = 1 or 2. The hydrolysis reaction of Zn(II) is evaluated and it is found that it results in the formation of Zn(OH)+and Zn(OH)2aq species, at pH >5.6. Zinc shows maximum percent extraction (75%) with the pH 4.7 at low organic ligand concentration (0.01M).
KEYWORDS:liquid-liquid Extraction; zinc; 3-methyl-quinoxaline-2-thione; nitrate medium; pH range; 2-6
Download this article as:| Copy the following to cite this article: Senhaji S. B, Elyahyaoui A. Liquid-Liquid Extraction of Zinc By 3-Methyl-Quinoxaline-2-Thione From Nitrate Medium. Orient J Chem 2015;31(3). |
| Copy the following to cite this URL: Senhaji S. B, Elyahyaoui A. Liquid-Liquid Extraction of Zinc By 3-Methyl-Quinoxaline-2-Thione From Nitrate Medium. Orient J Chem 2015;31(3). Available from: http://www.orientjchem.org/?p=10959 |
Introduction
Zinc is an important base metal involved in various applications in metallurgical, chemical and textile industries [1-5]. It is mainly recovered from primary sulphide concentrates. A part of zinc is also recovered from different secondary resources such as zinc ash, zinc dross, flue dusts of electric arc furnace and brass smelting, automobile shredder scrap, rayon industry sludge etc[7, 37].Wastewater effluents, solid industrial waste and sewage are considered as the main pathways of zinc to aquatic environment [3].With rising environmental awareness, the presence of heavy metals, in nature is severely controlled [6]. Although zinc in micro amount is an essential oligo-element for a healthy body, zinc excess can be harmful, and causes zinc toxicity [5]. Therefore, zinc is listed in dangerous substance, and contamination lists proposed respectively, by European Union Directive and the United States Environmental Protection Agency (USEPA)[6]. The World Health Organisation(WHO) and USEPA recommend respectively,3.0 and 5.0 mg/L as maximal acceptable concentrations of zinc in drinking water [6]. The effluent standards are set at 2 mg/L, and 2.5 mg/g, respectively for wastewater [4], and solid waste disposal[6]. Reducing the discharge of this element into surface water becomes crucial operation [4,7].For compliance with strict environmental regulation, developing process for the recovery of zinc from leach solutions, spent solution and effluents becomes increasingly important[8].Several methods are used to achieve this purpose such as chemical precipitation, liquid –liquid extraction, ion exchange, and adsorption. Among these methods, liquid-liquid extraction is the most promising process for the separation and recovery of metals from the complex and low metallic containing solutions, for being one of the most economical and practical processes[2, 9, 10].Some conventional treatment techniques are less efficient, especially when zinc concentration effluent is relatively low[4].
The extraction of zinc is often carried out using high molecular weight amines and/or phosphoric, (RO)2P02H,phosphonic, (RO)(R)P02H, and phosphinic, (R)2P02H, acid extractants, from sulfuric and chloride acids media[2,8, 11-19].The advantage of these mixing systems is due to ability of used reagents to combine complexation phenomenon and ion exchange process.
Recently, there has been an increasing interest in the development of chelating ion exchangers with the aminoacid groups to improve the extractability and selectivity of heavy metal ions [19].Among the chelating ligands, quinoxalines have foundextensive applications in coordination chemistrydue to ease of preparation, and as they can form stable complexes with most transition metal ions[21, 22, 38-39].
To remove zinc more efficiently and economically requires more extensive information on the interaction of this element, with various extractant ligands, and inorganic anions. HNO3is suitable for a digestion of concentrated organic samples.The aim of the present study is to examine the extraction of zinc in NO3– medium, with 3-methyl-2(1H) quinoxaline-thione which is a bifunctionalextanctant groups.The advantage of this process is that can combine complexing phenomena, ion-pair formation and ion exchange reactions.
Experimental
Reagents and synthesis of 3-methyl-2 (1H)-quinoxalinethione
A 0.1M HNO3 stock solution of 10-3MZn (II) is obtained by dissolution of known amount of Highly pure Zinc Nitrate Hexahydrate[(Zn(NO3)2,6H2O), 99.999 wt%)] in adequate volume of nitric acid(HNO3), . A solution of synthesized extractant is prepared with dissolution of 0.011M of 3-methyl-2 (1H) quinoxaline-thione(C9H8N2S) in toluene(C7H8). All reagents are of analytical grade and used without further purification.
The syhthesized of 3-methyl-2 (1H) quinoxalinethione LH is well described in literature [23, 24].Briefly, the condensation of o-phenylenediamine and ethyl pyruvate in distilled water acidified (by sulphuric acid) for one hourat room temperature, results in the formation of 1: 3-méthylquinoxalin-2 (1H), which is purified by re-crystallization.Reaction of obtained compound with P2S5in refluxing pyridine for four hours gives 2: 3-methyl-2 (1H)-quinoxalinethione(Rdt : 75%). (Figure 1.).
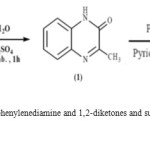 |
Figure 1 Click here to View figure |
Procedure
The organic and aqueous solutions were equilibrated in 20 ml separator funnel with an organic/aqueous ratio of 1:1 (5ml organic solution and 5ml aqueous solution) which is shaken vigorously for 15min. Preliminary tests have shown that equilibrium of extraction is reached in less than 5min. The starting pH of the aqueous phases,is adjusted by addition of drops of LiOH/HClO4 or KOH/HNO3, and measured before and after extraction test. After equilibration, the separator funnel is left standing for at least 10 min for completing phase separation. The aqueous zinc is then analyzed using Zincon (2-carboxy-2′-hydroxy-5′-sulfoformazylbenzene) spectrophotometrymethod[25, 26].Theextraction experiments are carried out in duplicate at room temperature, as a function of pH, and concentrations of nitrate ion and extractantdesignated there after as and , respectively. The zinc (II) concentration in the organic phase is obtained by mass balance. The distribution ratio, D, is calculated from the ratio of the equilibrium concentration of Zn(II) in the organic phase to that one in the aqueous phase.
The effect of the initial zinc concentration( ) on the extraction was studied in the range 0.001M– 0.2 M (Figure2). It was observed that the LH gives a better extraction for concentrations lower than 10-3M Zn (II).
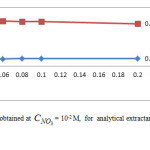 |
Figure 2 Click here to View figure |
Results and Discussion
Effect of equilibrium pH
The variation of logD versus pH obtained at = 10-2 M, for various analytical concentrations, , of chelatant are given in Figure 3. Similar variations with “S” shape are reported for the extraction of zinc and other divalent metals with some acidic extractants [27]. Distinct pH regions with different slopes characterize the various extracted species, and suggest the change in extraction mechanism.
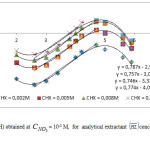 |
Figure 3 Click here to View figure |
As observed, extraction mechanism is less dependent on medium acidity for pH < 3. This process is ion-pair formation involving protonated amine group [20].
For pH > 3, the extraction mechanism is typical of ion exchange. As a result, logD increases with pH to reach a maximum at pHmax and then decreases as pH continues to rise. Optimum pH max is found at 4.7, in all explored CLH conditions.
When pH becomes higher than pH max (4.7)the functional groups with strongly acidic cation exchanger “sulphonate -SO3H” reach its maximum sorption capacity[20].
On the other hand the ternary amine in position 4 is protonated and sites of bonding to metal ions are limited en ternary amine in position 2 so the extraction is decreased [35].
At pH 5.6 and higher, zinc seems to exhibit a typical hydroxylated species extraction behavior [36]. Similar trend in removal efficiency with pH is reported on the extraction of Zn(II) and analogous divalent metals with organophosphorus extractants [12, 27]. The hydrolysis equilibrium can be expressed as: Zn2+hH2O↔Zn(OH)h(2-h)++hH+(3) If D0 designate the particular value of distribution coefficient in the absence of hydrolysis phenomenon, we obtain
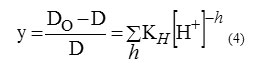
KHis the apparent hydrolysis equilibrium constant.
The computation of log D0 is performed on the basis of corresponding linear equation of log D =f(pH) (Figure 3.), obtained at CLH = 0.01 M and pH >5.6.Figure 4 shows that logy = f(pH) variations are linear with slope (h)varying in the range : 1.3 – 1.4. As a result for pH range: 5.6-6, the both first and second hydrolysis reactions are involved, in these conditions.
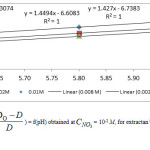 |
Figure 4 Click here to View figure |
Taking into consideration the molar fraction (x) of Zn(OH)+ specie, the overall hydrolysis equilibrium could be written as
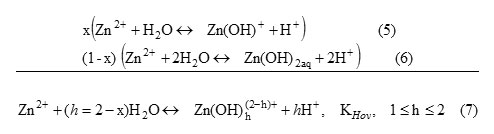
KHov is the overall hydrolysis constant.Based on theexperimental results (Figure 4-2), we obtain logKHov= -6.55 and x= 0.61. This indicates that Zn(OH)+ and Zn(OH)2aq are the hydrolyzed species formed in this case,and their molar fraction are 61% and 39%, respectively.
Effect of extractant concentration
As shown from logD=f(pH) study, the extraction of Zn(II) with 3-methyl-2 (1H)-quinoxalinethioneis a complex process involving, more than one extraction reaction. In order to have a better understanding of the extraction mechanism,further information is required in this case. For this reason, experimental analysis of the logarithmic variation of D with CLH, is undertaken at pH values ranging from 2 to 6.0 (Figure 5.).As found,straight lines with slopes close to 1.5 and 1.1 are obtained in the pH ranges of 1.3 – 5.0 and 5.6 – 5.9, respectively.So, the stoichiometry coefficient, l=
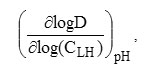
is not integer as might be expected from theoretical single reaction.Thus, for pH< 5 the extraction of zinc in nitrate medium, by 3-methyl-quinoxaline-2-thione, results in a combination of at least two predominant extracted complexes with1 (l =1) and 2 (l =2) extractant ligands. While for pH ≥ 5.6 the 1:1 metal-ligand complex is the prevailing extracted specie. Previous studies report that the metal: extractant ligand ratio of 1:1, and 1:2 is obtained for the extraction mechanisms of Zn(II) with amine and/ororganophosphoruschelatants[15,28].
 |
Figure 5 Click here to View figure |
Effect of nitrate concentration(CNO3)
The effect of nitrate anion added as NaNO3, is examined in 0.01M nitric acid solution (pH=2), for CLH= 0.01 and CNO3 ranging from 0.01 to 0.2 M. Obtained results are reported in Figure 6.
As it can be observed extraction of zinc by 3-methyl-2 (1H)-quinoxaline-2-thione increases with increasing CNO3 and reaches a maximum of 97% at CNO3 = 0.07M. The plots of logD=f (CNO3) (Figure 5) exhibits straight lines with different slopes, indicating that extraction reaction involves the formation of complexes with various Zn(II) : NO–3 molar ratio, as discussed thereafter.
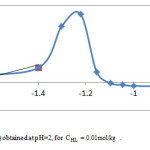 |
Figure 6 Click here to View figure |
Extraction reactions of Zn2+ with 3-methyl-quinoxaline-2-thione
It has been reported from previous study that zinc is extracted into chloroform with shift base extractants as both uncharged chelate complexes, and ion-pairs of charged species with a counter anionin aqueous solution[27].Taking into account the protonation of amino group at pH <3[20, 30], and neglecting the complexing effect of NO–3 [31], the extraction reaction of the predominant Zn2+ specie by 3-methyl-quinoxaline-2-thione symbolized as /LH in following, combinesion-pair formation and ionic exchange mechanisms. These reactions are expressed by the general equations.
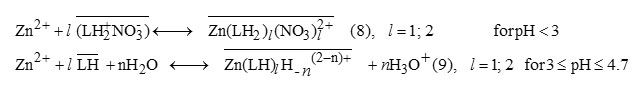
In the above equations, the onlined entities refer to organic phase andn= 0; 1; 2; etc. The symbol H-n stands both for hydrogen atoms (n< 0) and for OH group (n> 0).
The corresponding D expression being
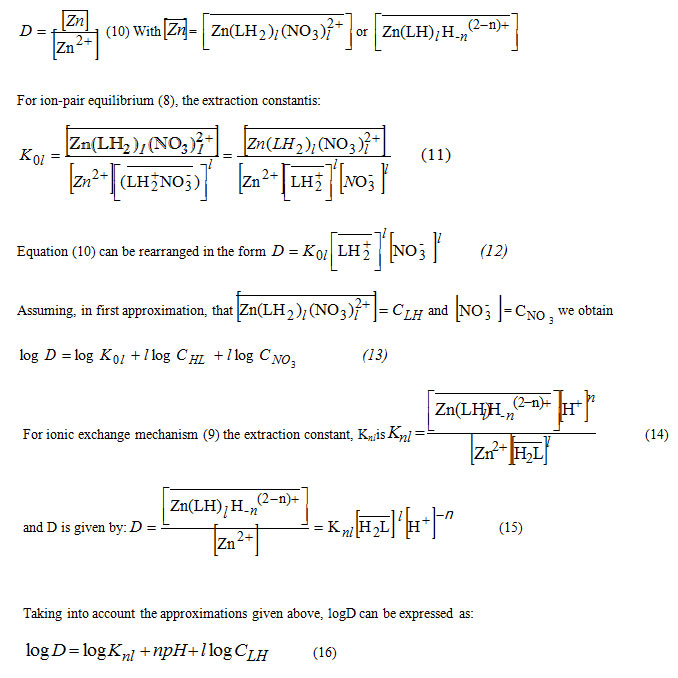
It appears that the distribution coefficient must be obtained in all cases, according to the general equation:
![]()
Wherem = land n=0 for pH< 3, and m= 0 and n≠ 0 in the pH range of 3≤ pH ≤ 5.6.
At given CNO3 and pH, equation (17) can be written as: logD= logA+IlogCHL (18)
![]()
Whereas at fixed CHL and pH, the expression of logD becomes
![]()
The exploitation of the distribution data on the basis of the general extraction equilibrium allows us to define the nature of the extracted complexes formed according to:
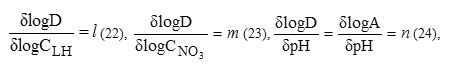
From results obtained at pH=2,log CHL=-2, and CNO3 ≤ 0.07M (Figure 6.), the straight line is of a slope ∫logD/∫logCNO3=m=1.4.This result is in consistent with that of logD=f(logCHL) (Figure 5.) obtained at pH≤ 5, showing l=1.5 which is comparable to m=1.4. The origin ordinate of obtained straight line which corresponds, in these conditions, to ion-pair mechanism,leads to logK01.5=7.42.
In addition, we have deduced from the experimental data logD=f (logCHL) (Figure 5), obtained at different pH values the variations of log A=f(pH) at CNO3 =10-2M (Figure 7). Straight lines having slopes of 0 and 1 are obtained respectively, at pH < 3 and 3 ≤ pH ≤ 4.7. These results are in agreement with those of Figure 2, indicating that Zn(II) is extracted with 3-methyl-quinoxaline-2-thione, essentially, by ion-pair formation in acidic medium (pH < 3), and by 1H+exchange reaction, in low acidic medium (3 ≤ pH ≤ 5). As discussed above, both ion-pair and ionic exchange reactions rise from a combination of at least two predominant reactions with l = 1 and 2.The apparent extraction constants, Kn1.5, could be determined by experiment, taking into account that m value. isof 1.4 and 0, respectively for pH < 3 and 3 ≤ pH ≤ 5.These constants are evaluated from intercepts of the logA=f(pH) straight lines allowing us to obtainlogK01.5 = 5.1, and logK11.5 = -0.6. As found,the logD=f(log CNO3) (Figure 5) and logA=f(pH) (Figure 6) straight lines obtained respectively at log CNO3 ≤ -1.4 and pH ≤ 3 have the same ordinate at the origins,confirming also that m=land n=0, because we have log CHO3 = log CHL = -2.
Taking into consideration the molar fraction (x) of extraction mechanism with 1 chelatant molecule, the overall partition equilibrium involving ion-pair formation, is summarized by the following reaction:
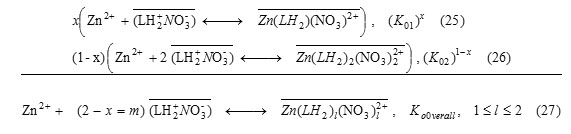
Knowing that the positively charged species could be neutralizedby NO–3 anion, as observed for other similar organic complexes [31], the reaction(27) becomes:
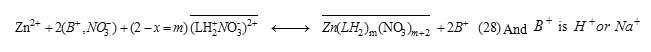
While the overall 1H+ exchange reactionis expressed as
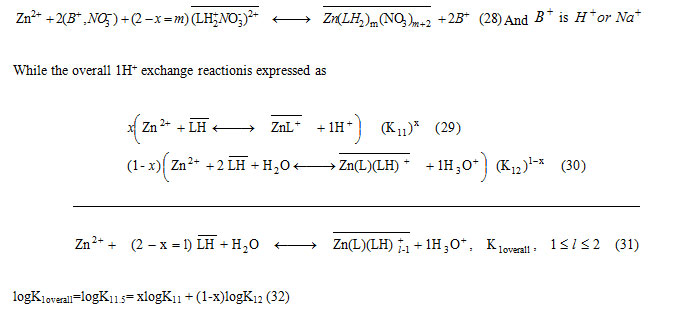
The molar fraction (x) can be obtained experimentally according to l= 1.5 = 2 – x for pH ≤ 4.7, in these conditions. Thus, the x values of 1:1 Zn-LH and 1:2 Zn-LH extracted species are of 0.5, in all cases. Accordingly, both ion-pair and ion exchange extracted complexes, involve 50% of Zn(LH2)2(NO3)2+ and Zn(LH2)2(NO3)22+ and ZnL+ and Zn(L)(LH)+ ,respectively. Similar complexes with 1:1 or 1:2 divalent metal-schift base ligandstoichiometry are reported in literature[29-30, 32-34].
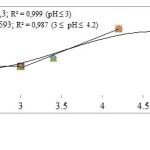 |
Figure 7 Click here to View figure |
Conclusion
Extraction of zinc from nitrate medium is carried out using 3-methyl-2 (1H)-quinoxalinethione in toluene. The extraction equilibrium of Zn(II) is examined as a function of pH, nitrate and chelate concentrations. The stoechiometryand the stability constants of the extracted Zn2+ species is postulated based on slope analysis method. Obtained results show that the extraction reaction involves both ion-pair formation and 1H+ exchange mechanism. The stoechiometry of the prevailing complexes are found to be 1:1 and 1:2 metal-ligand ratios, in all cases. The molar fraction of these complexes isevaluated. It is foundthat extracted species include 50% of each of identified complexes in all explored conditions. The formation of hydroxylated species is also considered. The formation of Zn(OH)+ and Zn(OH)2aq is shown to take place for pH >5.6, their molar fraction are 61% and 39%, respectively. In addition, extraction of zinc by 3-methyl-2 (1H)-quinoxaline-2-thione increases with increasing CNO3, and reaches a maximum of 97% at CNO3 = 0.07 M / pH=2.
References
- M.K. Jhaa, V. Kumar, D. Bagchi , R.J. Singh, J-C.Lee,; J. Hazard. Mater.2007, 145, 221-226.
- S.H .Hasan, M .Talat, S.Rai ;Technol. 2007,98, 918–928.
- JRC-IHCP : Zinc Metal Part I In: Environment, Risk Assessment Report, European Union Final report, European Commission, Joint Research Centre (JRC), Institute for Health and Consumer Protection (IHP), May 2008, Luxembourg: Publications Office of the European Union, (2010).
- Yamagata, H., Yoshizawa M, Minamiyama M. 2010. Assessment of current status of zinc in wastewater treatment plants to set effluent standards for protecting aquatic organisms in Japan. Environ. Monit. Assess.169 (2010) 67–73.
- S.A. Khan, Z.U. Din.; Int. J. Sci. Nat.2011, 2(3), 648- 652.
- G. Basak , V. Lakshmi ,C. Preethy , D. Nilanjana ; J. Environ. Health Sci. Eng. 2014, 12(8), 1-2 .
- FME.: Discharge of zinc, copper and lead to water and soil, Environmental Research of the federal Ministry of the environment , Nature Conservation and Nuclear Safety, Research report 20/02, Germany(2005), pp 202-242.
- H. L. Kwan, W.S. Geoff, E.K. Sandra; Hydrometallurgy. 2014, 142, 108–115.
- S.K. Sahu, V.K. Verma, D. Bagchi, V. Kumar , B.D. Pandey; Indian. J. Chem. Technol. 2008, 15, 397-402
- M. El Moujabber, L. Mandi, G. Trisorio-Liuzzi, I.Martín, A. Rabi, R. Rodríguez; Technological perspectives for rational use of water resources in the Mediterranean region. Bari. CIHEAM. Options Méditerranéennes : Série A. Séminaires Méditerranéens; N. 88 (2009) 187-198.
- S. Amer, J.M. Figueiredo, A. Luis ;Hydrometallurgy. 1995, 37(3), 323–337.
- D.D. Pereira, S.D. Ferreira Rocha, M.B. Mansur; Sep. Purif. Technol. 2006, 53(1) 1-8.
- Q.Jia, J.Wu, T-T.Li, W-H. Zhou; Chinese J. Anal. Chem. 2008, 36(5), 619–622.
- S.N. Patkar, M.T. Saeed , Z.H. Rizvi, J. Ahmad, M.Y. Shaheen; J. Pak. Inst. Chem. Eng. 2009, XXXVII, 1-13.
- S., X. Tong, Z. Naizhong , Q. Jia, W. Zhou, W. Liao,;. Hydrometallurgy. 2009, 100, 15–19.
- N. Song, S. Tong, W. Liu, Q. Jia, W. ., Zhoua, W. Liaob; J. Chem. Technol. Biotechnol. 2009,84, 1798–1802.
- L. Huai-zhong, C. Li-yuan, Q. Wen-qing, T. Shuang-hua; J. Cent. South Univ. Technol. 2010,17, 760−764.
- M. Tian, F. Mu, Q. Jia, X. Quan, W. Liao; J. Chem. Eng. Data.2011, 56, 2225–2229.
- M. Shamsi, E. Farah bakhsh; Recovery of zinc from effluent of plants and mines, 2nd Intl’ Conference on Advances in ngineering Sciences and Applied Mathematics (ICAESAM’2014) May 4-5, Istanbul (Turkey) (2014).
- D. browski , Z. Hubicki , P. Podko, E. Robens; Chemosphere. 2004, 56, 91–106
- Sebastian , M.: Transition metal complexes of quinoxaline based Schiff base ligands: Synthesis, characterization and catalytic activity study. PhD, Faculty of Science, Cochin University of Science and Technology, Kochi, Kerala, India. (2010)
- N. ShubhangiKotkar, D. HarjeetJuneja;E-J. Chem. 2013, 2013, 1-5.
- Cheeseman, G. W. H., Cookson , R. F.: Condensed pyrazines. In: Cheeseman, A., Taylor, E.C. (edts.). The chemistry of heterocyclic compounds.35. John Wiley & Sons, Inc., New Jersey (1979)
- Brown, D.: J. Primary Syntheses. In Quinoxalines: Supplement II, Volume 61, John Wiley & Sons, Inc., Hoboken, New Jersey (2004)
- ASTM D 1691-84 : Zinc in Water,Test Method A.. Standard Methods for the Examination of Water and Wastewater (Standard Methods), APHA .Method 3500-Zn B – 1997. 22. US EPA (2012).
- Method 8009, Zincon Method for Zinc. In: Hach Handbook of Water Analysis, pp.2-231. Hach Company, Lovel and (1979)
- Baba, A. A., Adekola, F. A.: Extraction of Zinc (II) by Triphenylphosphite (TPP) in Hydrochloric acid: kinetics and mechanism. Int. J. Phys. Sci. 3(4) (2008) 104-111.
- N. Hirayama, N. Ichitani, K. Kubono, Y. Matsuoka, H. Kokusen, T. Honjo ;Talanta. 1997, 44(0997) 2019-2025.
- R. Laus, A. dos Anjos, R. E. H. M. Osório, B. A. M. Neves, C. M. Laranjeira, V. T. de Fávere; Pak. J. Anal. Environ. Chem. 2008, 9(2) 58 – 63.
- B. Carla, B. Antonio, G. Claudia, G. Paola, M.Palma, V. Barbara; Dalton Trans.2013,42, 12130-12138.
- Sillen, L.G., Martell, A. E., Bjerrum, J.: Stability constants of metal-ion complexes. Chem. Soc 17. RSC, London (1964).
- M.S. Lee, S. Nam,;J. Korean Chem. Soc. 2009, 30, 7.
- A.A. Baba, F. A Adekola;.Journal of King Saud University– Science. 2013, 25, 297–305.
- A. M. Wilson, J. B. Phillip, A. T. Peter, R. T. Jennifer, A. G. Richard, Jason, B. L.; Chem. Soc. Rev. 2014, 43, 123-134.
- R. Laus, A. R. dos Anjos, E. H. M. B. Osório, A. Neves; Pak. J. Anal. Environ. Chem. 2008, 59(9), 2.
- T.W.J. Albrecht, J. Addai-Mensah, D. Fornasiero; Effect of pH, concentration and temperature on copper and zinc hydroxide formation/precipitation in solution. Chemeca 2011: Engineering a Better World: Sydney Hilton Hotel, NSW, Australia, 18-21 September 2011.
- D. BENOUALI, S. KHERICI, M. BELABBASSI; Orient. J. Chem. 2014, 30(2), 515-519
- K. KARIMNEZHAD, A. MOGHIMI; Orient. J. Chem.2014, 30(1), 95-103.
- D. VILLEMIN, M. A. DIDI ;Orient. J. Chem. 2013, 29(4), 1267-1284.

This work is licensed under a Creative Commons Attribution 4.0 International License.









