Imipramine Drug Delivery Via Swbnnts and Dwcnts: NMR and Solvent Effect
Azadeh Nazarian
Department of Chemistry, Science and Research Branch, Islamic Azad University, Tehran, Iran. Corresponding Author Email: Azade.nazarian.1988@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/310327
Article Received on :
Article Accepted on :
Article Published : 16 Sep 2015
The drug delivery via multi wall nano tube has been studied using ab-initio and QM/MM methods. We have studied the solvent effects on the relative energies and dipole moment values and the structural properties of water, methanol surrounding single-walled and multi walled carbon and boron nitride nanotubes. In this study we investigated the polar solvents effects on MWCNT within the Onsager self - consistent reaction field (SCRF) model using a Hartree-Fock method and the temperature effect on the stability of SWCNT in various. Because some of the physicochemical parameters related to structural properties of SWCNT, we used different force fields to determine energy and other types of geometrical parameters, on the particular SWCNT.
KEYWORDS:SWBNNT; DWCNTs; DFT; Thermodynamic; NMR; MWNTs; Imipramine
Download this article as:| Copy the following to cite this article: Nazarian A. Imipramine Drug Delivery Via Swbnnts and Dwcnts: NMR and Solvent Effect. Orient J Chem 2015;31(3). |
| Copy the following to cite this URL: Nazarian A. Imipramine Drug Delivery Via Swbnnts and Dwcnts: NMR and Solvent Effect. Orient J Chem 2015;31(3). Available from: http://www.orientjchem.org/?p=10941 |
Introduction
Imipramine is used in the treatment of depression, such as depression associated with agitation or anxiety. It is similar in efficacy to the antidepressant drug moclobemide [1-4]. It has also been used to treat nocturnal enuresis because of its ability to shorten the time of delta wave stage sleep, where wetting occurs [2-5].
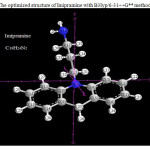 |
Figure 1: The optimized structure of Imipramine with B3lyp/6-31++G** method Click here to View figure |
In 1958, Dr Freyhan discussed about the effects of imipramine in a group of 46 patients, most of them were diagnosed as “depressive psychosis”. The patients were selected for this study based on symptoms such as depressive apathy, kinetic retardation and feelings of hopelessness and despair. In 30% of all patients he reported optimal results and in around 20% failure. The side effects noted were classified as atropine-like and most patients suffered from dizziness. Imipramine was first tried against psychotic disorders such as schizophrenia, but proved insufficient. As an antidepressant, it did well in clinical studies and it is known to work well in even the most severe cases of depression [4-6].
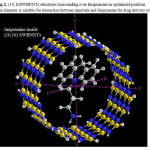 |
Figure 2: (10,10SWBNNTs structures surrounding over Imipramine in optimized position The diameter is suitable for interaction between nanotube and Imipramine for drug delivery system Click here to View figure |
Imipramine has additional indications for the treatment of panic attacks, chronic pain, and Kleine-Levin syndrome [3-6].
Imipramine may cause a high rate of manic and hypomanic reactions in hospitalized patients with preexisting bipolar disorder, with one study showing that up to 25% of such patients maintained on Imipramine switched into mania or hypomania [4-7]. Such powerful antidepressant properties have made it favorable in the treatment of treatment-resistant depression.
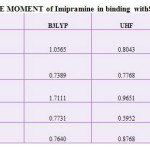 |
Table 1: DIPOLE MOMENT of Imipramine in binding with SWBNNTs Click here to View table |
It became extensively used as a standard antidepressant and later served as a prototypical drug for the development of the later-released tricyclics. It is not as commonly used today, but is sometimes used to treat major depression as a second-line treatment. It has also seen limited use in the treatment of migraines, ADHD, and post concussive syndrome.
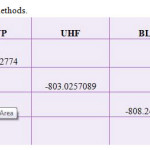 |
Table 2: Energy in various methods. Click here to View table |
The highly electrophilic central carbon atom of the -N=C=S group reacts rapidly, and under mild conditions with oxygen-, sulfur-, or nitrogen centered nucleophiles to give rise to carbamates, thiol-carbamates, or thiol-urea derivatives, respectively. The -NCS group of isothio-cyanates absorbs UV light with low intensity near 240 nm. Among the most extensively investigated isothio-cyanates is PEITC which occurs naturally as glucosinolate in a variety of cruciferous vegetables such as: kale, turnip, cabbage and broccoli. Phenethyl-ITC (PEITC) is one of the best-studied members of the ITC family of compounds that has generated a great deal of research interest due to its cancer chemo-preventive activity [1-9]. Angiogenesis (formation of new blood vessels): cancer, rheumatoid arthritis, endometriosis and diabetic retinopathy. Therefore, antiangiogenic therapy represents one of the most promising approaches to control tumor growth and invasiveness. PEITC inhibits transcriptional activity of nuclear factor-kB (NF-kB) and suppresses expression of NF-kB–regulated genes, including vascular endothelial growth factor Because VEGF plays an important role in angiogenesis by promoting endothelial cell proliferation, migration, and differentiation. PEITC effectively inhibits in vitro angio-genic features. PEITC effectively inhibited chemically-induced lung, mammary gland, and forestomach and esophagus tumorigenesis. Phenethyl isothio-cyanate exhibits anti-leukemic activity in vitro and in vivo [2-8].
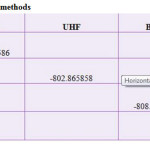 |
Table 3: NMR in various methods Click here to View table |
The carbon nanotube (CNT) is a representative nano-material. CNT is a cylindrically shaped carbon material with a nano-metric-level diameter [9-30].
Its structure, which is in the form of a hexagonal mesh, resembles a graphite sheet and it carries a carbon atom located on the vertex of each mesh. The sheet has rolled and its two edges have connected seamlessly [29-45].
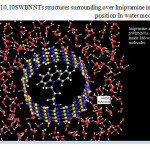 |
Figure 3: (10,10SWBNNTs structures surrounding over Imipramine in optimized position In water media Click here to View figure |
Although it is a commonplace material using in pencil leads, its unique structure causes it to present characteristics that had not found with any other materials. CNT can be classified into single-wall CNT, double-wall CNT and multi-wall CNT according to the number of layers of the rolled graphite [46, 70].
The type attracting most attention is the single-wall CNT, which has a diameter deserving the name of “nanotube” of 0.4 to 2 nanometers. The length is usually in the order of microns, but single-wall CNT with a length in the order of centimeters has recently released [31,40].
 |
Figure 4: Ω of Imipramine in binding with SWBNNTs Click here to View figure |
CNT can be classified into single-wall CNT, double-wall CNT and multi-wall CNT according to the number of layers of the rolled graphite. The type attracting most attention is the single-wall
CNT, which has a diameter deserving the name of “nanotube” of 0.4 to 2 nanometers [48-72]. The length is usually in the order of microns, but single-wall CNT with a length about centimeters have recently released. The extremities of the CNT have usually closed with lids of the graphite sheet [50-79].
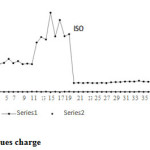 |
Figure 5: Iso versues charge Click here to View figure |
The lids consist of hexagonal crystalline structures (six-membered ring structures) and a total of six pentagonal structures (five-membered ring structures) placed here and there in the hexagonal structure [53-80]. The first report by Iijima was on the multiwall form, coaxial carbon cylinders with a few tens of nanometers in outer diameter [55-84]. Two years later single walled nanotubes were reported [59-93]. SWCNTs have considered as the leading candidate for nano-device applications because of their one-dimensional electronic bond structure, molecular size, and biocompatibility, controllable property of conducting electrical current and reversible response to biological reagents hence SWCNTs make possible bonding to polymers and biological systems such as DNA and carbohydrates [64-98].
 |
Table 4: Thermodynamic data in various solvent. Click here to View table |
So, The structure of SWCNT as well as their dipole moments and relative energies have been studied by molecular dynamics simulation and quantum mechanics calculations within the Onsager self – consistent reaction field (SCRF) model using a Hartree-Fock method (HF) at the HF/3-21G level and the structural stability of considered nanotube in different solvent media and temperature have been compared and analyzed. The calculations have been done with the GAUSSIAN 98 program according to Hartree-Fock (HF) theory at the HF/STO-3G level. Gibbs free energy, enthalpy, entropy and dipole moment values are compared in gas phase, water and methanol, in this research.
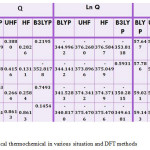 |
Table 5: staticitical thermochemical in various situation and DFT methods Click here to View table |
Computational Details
A method, which avoids making the HF mistakes in the first place, is called Quantum Monte Carlo (QMC). There are several flavors of QMC variational, diffusion and Green’s functions. These methods work with an explicitly correlated wave function and evaluate integrals numerically using a Monte Carlo integration. These calculations can be very time consuming, but they are probably the most accurate methods known today. In general, ab initio calculations give very good qualitative results and can give increasingly accurate quantitative results as the molecules in question become smaller.
The term “Ab Initio” is given to computations which are derived directly from theoretical principles, with no inclusion of experimental data. The most common type of ab initio calculation is called a Hartree-Fock calculation, in which the primary approximation is called the central field approximation.
 |
Table 6: NMR data in various situation Click here to View table |
DFT is based on a theorem due to Hohenberg and Kohn, which states that all ground state properties are functions of the total electronic charge density ρ(r).There are several different DFT functional available differing primarily in the choice of the basic functions, in which, the electronic wave functions are expanded and the scheme of integration.
The Becke’s three parameter exact exchange functional (B3) combined with gradient corrected correlation functional of Lee–Yang–Parr (LYP) have been employed to calculate energy, dipole moment, charge distribution and thermochemical data and NMR parameters by implementing the 6-31G,STO-3G basis sets. All the NMR shielding parameters were calculated supposing gauge-included atomic orbital (GIAO) method.
There are three steps in carrying out any quantum mechanical calculation in HyperChem 7.0 program package. First, prepare a molecule with an appropriate starting geometry. Second, choose a calculation method and its associated options. Third, choose the type of calculation with the relevant options.
NMR spectroscopy is a research technique that exploits the magnetic properties of certain atomic nuclei to determine physical and chemical properties of atoms or the molecules in which they are contained. It relies on the phenomenon of nuclear magnetic resonance and can provide detailed information about the structure, dynamics, reaction state, and chemical environment of molecules. Ab initio calculation of nuclear magnetic shielding has become an indispensable aid in the analysis of molecular structure and accurate assignment of NMR spectra of compounds.
NMR is based on the quantum mechanical property of nuclei. The chemical shielding refers to the phenomenon, which is associated with the secondary magnetic field created by the induced motions of the electrons that surrounding the nuclei when in the presence of an applied magnetic field. In general, the electron distribution around a nucleus in a molecule is more spherically symmetric. Therefore, the size of electron current around the field, and hence the size of the shielding, will depend on the orientation of the molecule within the applied field B0.
Calculations of nucleus-dependent and -independent chemical shifts were carried out using the gauge-invariant atomic orbital (GIAO) approach.
The chemical shielding tensor includes the chemical shift isotropy (CSI) and chemical shift anisotropy (CSA) and the anisotropy (Δб) of the tensor, the shielding tensor asymmetry parameter (η) and chemical shift (δ) are calculated.
The chemical shift refers to phenomenon which associated with the secondary magnetic field created by the induced motions of the electrons that surrounding the nuclei when in the presence of an applied magnetic field. .. The shielding σ is the differential resonance shift due to the induced motion of the electrons. The chemical shielding tensor is commonly referred to the chemical shift anisotropy (CSA) tensor according to the possession of second rank properties. The CSA tensor can be described by three additional parameters.
a) The isotropic value( ), of the shielding tensor which can be defined as: [59-61].
1
= 3 ( 11 + 22 + 33)
The chemical shift anisotropy parameter (∆σ), due to the following expression:
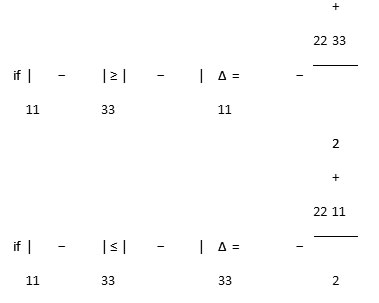
The asymmetry parameter (ƞ), which has given by:
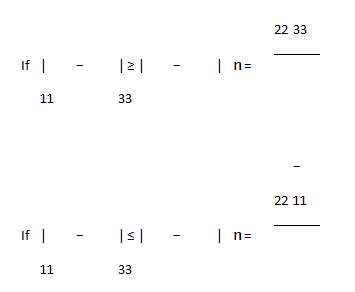
It is useful to define the span (Ω), and the skew (ĸ) of a CSA tensor. The span is defined as:
= 33 − 11
And indicates the width of the NMR line shape for a nonspinning, stationary, sample . The skew is defined as:
ĸ = 3( − 22)
Calculations were performed using an all-electron linear combination of atomic orbitals Hatree– Fock (HF) and density functional theory (DFT) calculations using the Gaussian 03 package. The optimizations of isolated imipramine and imipramine inside the SWBNNTS and DWCNNTs are carried out including exchange and correlation contributions using Beckẻs three parameter hybrid and Lee-Yang-Parr (LYP) correlation [B3LYP]; including both local and non-local terms. We have geometric optimization calculation at the HF/6-31G, HF/6-31G**, HF/6-311G**. We have also performed a geometric optimization calculation at the B3LYP/6-31G, B3LYP/6-31G** and B3LYP/6-31G** level. The NMR isotropic shielding constants were calculated using the standard GIAO (Gauge-Independent Atomic Orbital) approach of Gaussian 03 program package.
Langevin dynamics (LD) Simulation
The molecular dynamics method is useful for calculating the time dependent properties of an isolated molecule. However, more often, one is interested in the properties of a molecule that is interacting with other molecules.
The Langevin equation is a stochastic differential equation in which two force terms have been added to Newton’s second law to approximate the effects of neglected degrees of freedom .These simulations can be much faster than molecular dynamics.
Monte Carlo simulation
The Metropolis implementation of the Monte Carlo algorithm has been developed by studying the equilibrium thermodynamics of many-body systems. This is understandable because the Monte Carlo simulations always detect the so-called “important phase space” regions which are of low energy. Because of imperfections of the force field, this lowest energy basin usually does not correspond to the native state in most cases, so the rank of native structure in those decoys produced by the force field itself is poor.
Choosing small trial moves, the trajectories obtained applying this algorithm agree with those obtained by Langevin’s dynamics
In density function theory the exact exchange (HF) for a single determination is replaced by a more general expression the exchange correlation functional, which can include terms accounting for both exchange energy and the electron correlation, which is omitted from Hartree-Fock theory: Eks =n + hp +1/ 2 Pj (r) + Ec (r ) + EC(r )
Where Ec (r ) is the exchange function and EC (r ) is the correlation functional.
Results and Discussion
We used different force field to determination of energy and other type of geometrical parameters, on the particular SWBNNT, and DWCNTs Because of the differences among force fields, the energy of a molecule calculated using two different force fields will not be the same. In the process of investigating the combination of particular molecules and CNT to achieve more information about these important gene transfer systems, we attempted to construct different situation of binding Imipramine to SWBNNTs and DWCNTs for linking them individually to outer and inner surface of tubes to develop practical application of these molecules to interact with SWBNNTs and DWCNTs.
So, it is not reasonable to compare the energy of one molecule calculated with a particular force field with the energy of another molecule calculated using a different force field. In this study difference in force field illustrated by comparing the energy of calculated by using force fields, MM+, Amber and OPLS. Theoretical energy values using different force fields, which is the combination of attraction van der Waals forces due to dipole-dipole interactions and empirical repulsive forces due to Pauli repulsion has been demonstrated in Table1. Since the solute dipole moment induces a dipole moment in opposite direction in the surrounding medium.
Also, polarization of the medium in turn polarizes the charge distribution in the solvent. The dipole moment value of SWCNT in different solvent media at same temperatures has been reported.
References
- Heck, HA; Buttrill, JE Jr; Flynn, NW; Dyer, RL; Anbar, M; Cairns, T; Dighe, S; Cabana, BE. Journal of Pharmacokinetics and Biopharmaceutics June .1979, 7 (3), 233–248
- Lepola, U.; Arató, M.; Zhu, Y.; Austin, C. J Clin Psychiatry.2003, 64 (6), 654–62
- Delini-Stula, A.; Mikkelsen, H.; Angst, J. J Affect Disord, 1995, 35, (1–2), 21–30
- Bottlender, R.; Rudolf, D.; Strauss, A.; Möller, HJ.; European Archives of Psychiatry and Clinical Neuroscience ,1998, 248, (6), 296–300
- Tsankova, N.M. Berton, O.; Renthal, W.; Kumar, A.; Neve, RL. Nestler, EJ.Nature Neuroscience, 2006, 9 (4), 519–25
- Krishnan, V.; Nestler, EJ. Nature, 2008, 455, (7215), 894–902
- de Gandarias, JM.; Echevarria, E.; Acebes, I.; Silio, M.; Casis, L. Arzneimittel-Forschung, 1998, 48 (7): 717–9
- Brunton, L.; Chabner, B.; Knollman, B. Goodman and Gilman’s The Pharmacological Basis of Therapeutics, Twelfth Edition. McGraw Hill Professional, 2010.
- Skidmore-Roth, L.; (Ed.). Mosby’s Nursing Drug Reference (23rd ed.). St. Louis, MO: Mosby Elsevier (2010).
- Paganelli CV .Solomon AK .J Gen Physiol .1957, 41, 259-277
- Monajjemi, M.; Lee, V.S.; Khaleghian, M.; B. Honarparvar, B.; F. Mollaamin, F. J. Phys.Chem. C. 2010, 114, 15315
- Whittembury G. J Gen Physiol.1960, 43, 43-56
- Monajjemi, M. Struct Chem. 2012, 23,551–580
- Monajjemi, M.; Chegini, H.; Mollaamin, F.; Farahani, P. Fullerenes, Nanotubes, and Carbon Nanostructures.2011, 19, 469–482
- Monajjemi, M .; Afsharnezhad ,S.; Jaafari , M.R.; Abdolahi ,T.; Nikosade ,A.; Monajemi ,H.; Russian Journal of physical chemistry A, 2007, 2,1956-1963
- Monajjemi, M.; Baei, M.T.; Mollaamin, F. Russian Journal of Inorganic Chemistry. 2008, 53 (9), 1430-1437
- Farmer RE.; Macey RI. BiochiBiophys Acta, 1970, 196, 53-65.
- Monajjemi, M.; Rajaeian, E.; Mollaamin, F.; Naderi, F.; Saki, S. Physics and Chemistry of Liquids. 2008, 46 (3), 299-306
- Monajjemi, M.; Boggs, J.E. J. Phys. Chem. A, 2013, 117, 1670 −1684
- Mollaamin, F.; Monajjemi, M, Journal of Computational and Theoretical Nanoscience. 2012, 9 (4) 597-601
- Sidel, VW. Solomon AK. J Gen Physiol .1957, 41, 243-257.
- Moura TF. Macey, RI. Chien DY.; Karan D.; Santos H. J Member Biol. 1984, 81, 105-111.
- Monajjemi, M.; Khaleghian, M, Journal of Cluster Science. 2011, 22 (4), 673-692
- Mollaamin, F.; Varmaghani, Z.; Monajjemi, M, Physics and Chemistry of Liquids. 2011, 49 318
- Nafisi, S.; Monajemi, M.; Ebrahimi, S. Journal of Molecular Structure. 2004,705 (3) 35-39
- Preston GM. Carroll TP.; Guggino W.B.; Agre P. Science. 1992, 256, 385-387.
- PAO, G. M.; WU, L. F.; JOHNSON, K. D.; HOFTE, H.; CHRISPEELS, M. J.; SWEET, G.; SANDAL, N. N.; SAIER JR.z M. H. Molecular Microbiology.1991. 5, 33–37
- WISTOW, G. J.; PISANO, M. M.; CHEPELINSKY, A. B.; Trends in Biochemical Sciences. 1991. 16, 170–171.
- Karkouri, El. K.; Gueune H.; Delamarche C. 2005. Biol Cell 97, 535-543.
- Zardoya R .Biol Cell. 2005, 97, 397-414.
- Fazaeli, R.; Monajjemi, M.; Ataherian, F.; Zare, K. Journal of Molecular Structure: THEOCHEM.2002, 581 (1), 51-58
- Monajjemi, M.; Razavian, M.H.; Mollaamin,F.; Naderi,F.; Honarparvar,B.; Russian Journal of Physical Chemistry A , 2008 , 82 (13), 2277-2285
- Monajjemi, M.; Seyed Hosseini, M.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures. 2013, 21, 381–393
- Monajjemi, M.; Faham, R.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures, 2012 20, 163–169
- Mollaamin, F.; Najafi, F.; Khaleghian, M.; Khalili Hadad, B.; Monajjemi, M. Fullerenes, Nanotubes, and Carbon Nanostructures, 2011 19, 653–667
- Menájem, M.; Mandaban, L.; Mollaamin, F.; Honarparvar, B. Fullerenes, Nanotubes and Carbon Nanostructures, 2010, 18, 45–55
- Iijima, Sumio. Nature (London), 1991. 354, 56.
- Chopra, Nasreen G.; Luyken , R. J.; Cherrey , K.; Crespi , Vincent H.; Cohen, Marvin L.; Louie, Steven G.; Zettl , A. Science,1995, 269, 966.
- Mollaamin, F.; Baei, MT.; Monajjemi, M.; Zhiani, R.; Honarparvar, B.; Russian Journal of Physical Chemistry A, Focus on Chemistry, 2008, 82 (13), 2354-2361
- Monajjemi, M. Chemical Physics. 2013, 425, 29-45
- Monajjemi, M.; Heshmat, M.; Aghaei, H.; Ahmadi, R.; Zare, K. Bulletin of the Chemical Society of Ethiopia, 2007, 21 (1)
- Monajjemi, M.; Honarparvar, B. H. ; Haeri, H. ; Heshmat ,M.; Russian Journal of Physical Chemistry C. 2006, 80(1):S40-S44
- Monajjemi, M.; Ketabi, S.; Amiri, A. Russian Journal of Physical Chemistry, 2006, 80 (1), S55-S62
- Yahyaei, H.; Monajjemi, M.; Aghaie, H.; K. Zare, K. Journal of Computational and Theoretical Nanoscience. 2013, 10, 10, 2332–2341
- Mollaamin, F.; Gharibe, S.; Monajjemi, M. Int. J. Phy. Sci, 2011, 6, 1496-1500
- Monajjemi, M.; Ghiasi, R.; Seyed Sadjadi, M.A. Applied Organometallic Chemistry,2003, 17, 8, 635– 640
- Monajjemi, M.; Wayne Jr, Robert. Boggs, J.E. Chemical Physics. 2014, 433, 1-11
- Monajjemi, M.; Sobhanmanesh, A.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures, 2013, 21 47–63
- Monajjemi, M.; Mollaamin, F. Journal of Computational and Theoretical Nanoscience, 2012, 9 (12) 2 208-2214
- Monajjemi, M.; Honarparvar, B.; Nasseri, S. M. .; Khaleghian M. Journal of Structural Chemistry. 2009, 50, 1, 67-77
- Monajjemi, M.; Aghaie, H.; Naderi, F. Biochemistry (Moscow).2007, 72 (6), 652-657
- Ardalan, T.; Ardalan, P.; Monajjemi, M. Fullerenes, Nanotubes, and Carbon Nanostructures, 2014, 22: 687–708
- Mollaamin, F.; Monajjemi, M.; Mehrzad, J. Fullerenes, Nanotubes, and Carbon Nanostructures.2014, 22: 738–751
- Monajjemi, M.; Najafpour, J.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures. 2013, 21(3), 213–232
- Monajjemi, M.; Karachi, N.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures, 2014, 22: 643–662
- Yahyaei, H.; Monajjemi, M. Fullerenes, Nanotubes, and Carbon Nanostructures.2014, 22(4), 346– 361
- Monajjemi, M. Falahati, M.; Mollaamin, F.; Ionics, 2013, 19, 155–164
- Monajjemi, M.; Mollaamin, F. Journal of Cluster Science, 2012, 23(2), 259-272
- Tahan, A.; Monajjemi, M. Acta Biotheor, 2011, 59, 291–312
- Lee, V.S.; Nimmanpipug, P.; Mollaamin, F.; Kungwan, N.; Thanasanvorakun, S..; Monajjemi, M.Russian Journal of Physical Chemistry A, 2009, 83, 13, 2288–2296
- Monajjemi, M.; Heshmat, M.; Haeri, HH, Biochemistry (Moscow), 2006, 71 (1), S113-S122
- Mollaamin, F.; Najafpour, J. ; Ghadami, S. ; Ilkhani, A. R. ; Akrami, M. S. ; Monajjemi, M. J.Comput. Theor. Nanosci. 2014, 11(5), 1290-1298
- 60. Monajjemi, M.; Yamola, H.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures, 2014, 22, 595–603
- Mollaamin, F.; Layali, I.; Ilkhani A. R.; Monajjemi, M. African Journal of Microbiology Research .2010, 4(24) 2795-2803
- Mollaamin, F.; Shahani poor, p K. .; Nejadsattari, T. ; Monajjemi, M. African Journal of Microbiology Research. 2010, 4(20) 2098-2108
- Monajjemi, M.; Ahmadianarog, M. Journal of Computational and Theoretical Nanoscience. 2014, 11(6), 1465-1471
- Monajjemi, M.; Jafari Azan, M.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures. 2013, 21(6), 503–515
- Mollaamin, F.; Monajjemi, M. Physics and Chemistry of Liquids .2012, 50, 5, 2012, 596–604
- Monajjemi, M.; Khosravi, M.; Honarparvar, B.; Mollaamin, F.; International Journal of Quantum Chemistry, 2011, 111, 2771–2777
- Macey RI; Karan DM; Farmer RE .1972, Biomembranes 3, 331-340.
- A. Rubio.; J.L. Corkill.; M.L. Cohen.; Phys. Rev. B ,1994, 49, 5081
- Khaleghian, M.; Zahmatkesh, M.; Mollaamin, F.; Monajjemi, M. Fullerenes, Nanotubes, and Carbon Nanostructures, 2011, 19(4): 251–261
- Monajjemi, M.; Baheri, H.; Mollaamin, F. Journal of Structural Chemistry.2011 52(1), 54-59
- Mahdavian, L.; Monajjemi, M.; Mangkorntong, N.Fullerenes, Nanotubes and Carbon Nanostructures, 2009, 17 (5), 484-495
- Monajjemi, M., Mahdavian, L., Mollaamin, F. Bull. Chem. Soc. Ethiop. 2008, 22(2), 277-286
- Monajjemi, M.; Afsharnezhad, S, Jaafari, M.R..; Mirdamadi, S..; Mollaamin, F..; Monajemi, H. Chemistry .2008, 17 (1), 55-69
- Monajjemi, M.; Mollaamin, F.; Gholami, M. R.; Yoozbashizadeh, H.; Sadrnezhaad, S.K.; Passdar, H.; Main Group Metal Chemistry, 2003, 26, 6, 349-361
- Monajjemi, M.; Azad ,MT.; Haeri, HH.; Zare, K.; Hamedani, Sh.; JOURNAL OF CHEMICAL RESEARCH-S.2003, (8): 454-456
- Monajjemi, M.; Najafpour, J. Fullerenes, Nanotubes, and Carbon Nanostructures, 2014, 22(6): 575– 5
- X. Blasé.; A. Rubio.; S.G. Louie.; M.L. Cohen.; Europhys. Lett, 1994.28, 335.
- N.G. Chopra.; J. Luyken.; K. Cherry.; V.H. Crespi.; M.L. Cohen,S.G. Louie.; A. Zettl, Science.1995 , 269, 966
- N.G. Chopra .;A. Zettl.;Solid State Commun.1998 ,105, 297
- Monajjemi, M.; Noei, M.; Mollaamin, F. Nucleosides, Nucleotides and Nucleic Acids. 2010 29(9):676–683
- Ghiasi, R.; Monajjemi, M. Journal of Sulfur Chemistry .2007, 28, 5, 505-511
- Monajjemi, M.; Ghiasi, R.; Abedi, A. Russian Journal of Inorganic Chemistry.2005, 50(3), 382-388
- Monajjemi, M. .; Naderi, F.; Mollaamin, F.; Khaleghian, M. J. Mex. Chem. Soc. 2012, 56(2), 207-211
- Monajjemi, M.; Farahani, N.; Mollaamin, F. Physics and Chemistry of Liquids, 2012, 50(2) 161–172
- Monajjemi, M.; Seyed Hosseini, M. Journal of Computational and Theoretical Nanoscience .2013, 10 (10), 2473-2477
- Monajjemi , M.; Honaparvar , B.; Khalili Hadad ,B.; Ilkhani ,AR.; Mollaamin, F. African Journal of Pharmacy and Pharmacology .2010, 4(8), 521 -529
- Monajjemi, M. Theor Chem Acc, 2015, 134:77 DOI 10.1007/s00214-015-1668-9
- Monajjemi, M. Journal of Molecular Modeling , 2014, 20, 2507
- Monajjemi , M.; Honarparvar, B.; Monajemi, H.;. Journal of the Mexican Chemical Society, 2006, 50 (4), 143-148
- J. Cumings.; A. Zettl, Solid State Commun.2004, 129, 661
- R. Ma.; Y. Bando.; H. Zhu.; T. Sato, C. Xu, D. Wu, J. Am.Chem. Soc.2002, 124, 7672,
- P.W. Fowler, K.M. Rogers, G. Seifert, M. Terrones, and H. Terrones, Chem. Phys. Lett. 1999, 299, 359
- Jalilian, H.; Monajjemi, M. Japanese Journal of Applied Physics. 2015, 54, 085101
- Monajjemi, M.; Khaleghian, M.; Mollaamin, F. Molecular Simulation. 2010, 36, 11, 865–870
- Naghsh, F, oriental journal of chemistry, 2015, 31(1)
- Chitsazan, A, oriental journal of chemistry, 2015, 31(1)

This work is licensed under a Creative Commons Attribution 4.0 International License.









