Heterogenous Photocatalysis Treatement of Azo Dye Methyl Orange by Nano Composite Tio2/Diatomite
Rachida Cherrak 1, 2, Mohammed Hadjel1, Noureddine Benderdouche3
1Laboratory of Science, Technology and Process Engineering (LSTGP), University of Science and Technology of Oran MB, El mnaouar BP, 1505, 31000 Oran, Alegria Mobile
2 University of science Abd el Hamid Ben Badis INES Mostaganem Algeria. Bp128 / 227, 27000 Mostaganem Algeria.
3Laboratory: structure, development and application of molecular materials (Sea2m)
Corresponding Author Email: cher.rachida@yahoo.fr
DOI : http://dx.doi.org/10.13005/ojc/310340
Article Received on :
Article Accepted on :
Article Published : 12 Sep 2015
The objective of this work is to study the removal of an azo dye methyl orange (MeO) by dioxide titanium supported on the surface of diatomite, as a new nano-composite by an advanced oxidation method as heterogeneous Photocatalysis. The titanium oxide (Degussa-25) was immobilized on the powder of diatomaceous earth with a very simple method and low expensive. Diatomite used in this study has porosity more 72%; was thermal activated at temperatures of 800 ° c and 900 ° c and 1000 ° C for 2 h and chemically by sulfuric acid at reflux. Photocatalytic degradation of methyl orange use, was studied in the presence of the materials prepared in solution aqueous with different compositions, M1 (1 g diatomite + 0.5 g TiO2) and M2 (5 g diatomite + 0.5 g TiO2). The photocatalytic activity of the prepared catalysts was tested in a single reactor followed by pH analyzes conductivity and the absorbance. The prepared materials exhibit a very porous morphology, which has been confirmed by several methods DRX, SEM and IR. The results of the photocatalytic treatment of water synthetically polluted with MeO at initial concentration 10 ppm showed a good performance for four nano composite prepared: M1TA is composed by material M1 with diatomite treated by sulfuric acid, and M1TT is composed by material M1 with diatomite calcined at 1000 ° C, and M2TA is M2 material with diatomite treated by sulfuric acid, and M2TT is M2 material with diatomite calcined at 1000 ° c. Maximum efficiency of removing MeO that reaches 84% and 72% for M2TA, M1TT According to the kinetic study reveals that the phenomenon is mixed resulting in a rapid response that is established after 30 minutes, the reaction kinetics of the methyl orange photodegradation following the model of the first order.
KEYWORDS:kieselguhrs; Titanium Dioxide; Photocatalysis; methyl orange
Download this article as:| Copy the following to cite this article: Cherrak R, Hadjel M, Benderdouche N. Heterogenous Photocatalysis Treatement of Azo Dye Methyl Orange by Nano Composite Tio2/Diatomite. Orient J Chem 2015;31(3). |
| Copy the following to cite this URL: Cherrak R, Hadjel M, Benderdouche N. Heterogenous Photocatalysis Treatement of Azo Dye Methyl Orange by Nano Composite Tio2/Diatomite. Orient J Chem 2015;31(3). Available from: http://www.orientjchem.org/?p=10826 |
Introduction
The treatment of colored wastewaters produced by the textile industry is a problem that has recently been heavily researched, [1, 2, and 3]. Heterogeneous Photocatalysis is one method for treatment of such wastewater; it is a so-called “Advanced Oxidation Process” (AOP) suitable for the oxidation of dyes.[4,5,6]. Titanium oxide is the most investigated photocatalyst for the degradation of organic pollutants from wastewaters. This catalyst is advantageous over other semiconductors because of its chemical stability, non-toxicity, low cost and commercial availability [7, 8, 9, and 10]. The efficiency of TiO2 is influenced by its crystal structure, particle size, specific surface area and porosity. Ultrafine powders of TiO2 show a good catalytic activity. These mineral impregnated with TiO2 show high thermal stability and larger pore sizes that afford better incorporation of the species without diffusion problems, increased the specific surface area [11, 8].Photocatalytic degradation of MeO was studied with diatomite/TiO2.
 |
Figure1: Molecular structure of methyl orange dye of textile Click here to View figure |
A synergetic effect of the porous minerals on the photocatalytic degradation due the large specific surface area was observed [12]. Studied the role of the porous minerals as supports of the TiO2 [13]. The authors examined the catalytic properties of the composites for degradation of methyl orange in aqueous solution. This pollutant was degraded at higher efficiency than by TiO2 (80%anatase + 20% rutile). Many techniques have been developed for the immobilization TiO2 catalysts onto a solid substrate. For example: Dip coating from suspension [14, 15], Spray coating and sol-gel. Also, different types of substrates have been tested. For example: Glass beads [16], glass tubes [17], fiber glass [18], quartz [19] stainless steel [20], aluminum [21], activated carbon [22], silica [23], and the glass plates [24], coal-fired [25], zeolithes [26,27].and TiO2 coated non magnetic material [28]. This work presents the preparation and characterization of titanium supported on diatomite ad the photodegradation of methyl orange.
Material and Methods
Preparation of samples
A simple and effective method has been used in this study for the immobilization of TiO2 nano particles which is ideal for industrialization. The same technique was applied with minor modifications for the preparation of the TiO2 coating on diatomite powders and used as photo catalysis.
The details of the technique and equipment are as follows
In the first step, 0.5 gram of titanium dioxide powder (Degussa P-25) is added to 18 ml of ethanol as the base medium of the slurry in which the titanium powder can be properly dispersed.
As soon as the titanium powder is added, it can be seen that the powder disperses easily through the Ethanol in a cloud-shape manner producing slurry.
In the second step, 1.5 ml of dilute nitric acid with pH of 3.5 is added to slurry. As the acid is added, the slurry becomes more uniform and the cloudiness start to disappear. The prepared suspension is used for the immobilization process of TiO2.
After the suspension was prepared, 1 gram then 5 gram of diatomite powder (washed with distilled water for few minute) and added to the suspension. Since diatomite powder is highly porous. It can naturally act as a good adsorbent and adsorb as much suspension as possible. Suggested contact time for slurry adsorption by diatomite powders is 30 minutes. It is recommended to mix powders with diatomite slurry during this period of time.
Finally the mud is calcined at 450 ° C for 30 minutes. The calcinations allow the titanium adhere more strongly to the pores of the powder of diatomaceous earth. When the coating process is completed and the diatomite powder is sufficiently cooled, it is washed with water for a few minutes to remove the titanium which is not attached correctly to the diatomite powder’s pores. This is how the new materials are obtained.
Table 1: the quantities TiO2 and diatomite used in the preparation of materials:
|
TiO2 |
DIATOMITE |
|
|
M1 |
0.5 gram |
1 gram |
|
M2 |
0.5 gram |
5 grams |
The Manipulations were performed in a reactor at room temperature (20-25 ° C) and a stirring speed of 600 r / min and pH close to 6, For this protocol we used four beakers of 250ml were introduced in all beakers of 200 ml MeO (10 mg / l) and then added to the first beaker 1 g M1TA,and in the second 1g of M1TT then 1g M2TA in the third, the fourth 1g M2TT, then we get all these beakers stirring in the dark room under UV radiation (wavelength of the light is 365 nm) in a reactor (fig.1). The Photocatalytic test was analyzed using a simple reactor with UV-VIS light absorption of light. The detector absorbance at 471 nm is set for methyl orange (MeO).
Samples of 20 ml solutions every 15 minutes measuring pH, conductivity then the sample is filtered through a paper membrane 0.45μm for finally, measure the absorbance by a UV-VIS spectrophotometer.
The Manipulations were performed in a reactor at room temperature (20-25 ° C) and a stirring speed of 600 r / min and pH close to 6, For this protocol we used four beakers of 250ml were introduced in all beakers of 200 ml MeO (10 mg / l) and then added to the first beaker 1 g M1TA,and in the second 1g of M1TT then 1g M2TA in the third, the fourth 1g M2TT, then we get all these beakers stirring in the dark room under UV radiation (wavelength of the light is 365 nm) in a reactor (fig.1). The Photocatalytic test was analyzed using a simple reactor with UV-VIS light absorption of light. The detector absorbance at 471 nm is set for methyl orange (MeO).
Samples of 20 ml solutions every 15 minutes measuring pH, conductivity then the sample is filtered through a paper membrane 0.45μm for finally, measure the absorbance by a UV-VIS spectrophotometer.
Structural characterization of the catalyst diatomite and TiO2
Prepared catalysts based on diatomite was determined using a scanning electron microscope (SEM) type 440i Leo. The quality of the coatings from the structural point of view, namely the P-25 crystal, 20% rutile and 80% anatase, underwent a significant change in their structures. The X-ray diffraction (XRD) of the obtained samples was performed in the laboratory materials at the Oran University Bruker DRX type D4 with a source of Cu Ka X-ray. Analysis infrared spectroscopy has been created by a device type Spectrum one “Perkin Elmer” 0.03 0005 gr KBr sample.
The photocatalytic experience
In ordrer to evaluate the photocatalytic efficiency of the prepared catalysts, the photocatalytic degradation of this MeO in water, was subjected to illumination by UV light. The immobilized catalysts on the powder diatomite were paid directly to the solution of MeO (10 mg / l) separately. The solutions were placed on a shaker and under a UV radiation source located 7 cm above the solution.
Samples from this experiment were filtered at 0.45 μm Millipore before being analyzed UV sources used in all experiments by the same medium pressure lamp (Osram) is 125 W.
Results and Discussion
Diatomite is a material having a high porosity exceeding 72% [29]. The morphological observations using a scanning electron microscope are shown in Figure 2, shows that in the images (a) raw diatomite and (b) shows the diatomite with good porosity after sulfuric acid treatment and in the image(c) heat treated diatomite 800 ° C and in the image (d) heat treated diatomite has 900 ° C and in the image (e) the heat treated diatomite 1000 ° C.
The main chemical components of diatomite analysis by X-ray fluorescence (XRF) are:
67.32% SiO2, 19.11% CaCO3, 1.91% Al2O3, 1.63% FeO3, 1.32% MgO, 0.75% K2O, 1.12%, Na2O, 1. 7% TiO2, 5.63% H2O.
Table 2: characterization result of the Algerian diatomite (SIG)
| pH |
7.9 |
| Pore diameter |
200-2000 |
| total porosity |
72% |
| permeability |
‹2% |
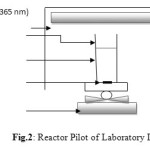 |
Figure 2: Reactor Pilot of Laboratory LSTGP. Click here to View figure |
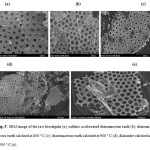 |
Figure3: SEM image of the raw kieselguhr (a); sulfuric acid treated diatomaceous earth (b); diatomaceous earth calcined at 800 ° C (c); diatomaceous earth calcined at 900 ° C (d); diatomite calcined at 1000 ° C (e). Click here to View figure |
The images show that the chemical treatment with sulfuric acid of the raw diatomite causes a significant change in its specific surface, as well as its pore volume distribution, may have noted that the porous particles are mainly in the form of diatomite circulaire.The pictures after treatment confirmed the high porosity, which make it a good support of the titanium dioxide.
After calcinations, specifically to 1000 ° C, there is a new distribution of pore volume and enlarging the diameter of the porous particles. This can be explained by the removal of organics impurities.
X-ray diffraction analysis
The graph of the XRD analysis are given in Figure
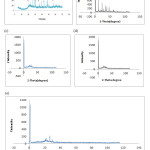 |
Figure 4: XRD of the raw diatomite DB (SIG) (a) sulfuric acid treatment diatomaceous earth (b), heat treatment diatomite at 800 ° C (C) heat treatment diatomite at 900 ° C (d) treatment heat diatomite at 1000 ° C (e). Click here to View figure |
The XRD analysis showed that diatomite (SIG) contains three crystalline phases: SiO2 (in the form of quartz), CaCO3 (in the form of calcite and dolomite) and amorphous SiO2. However the clay phases were not found in this unlike many other diatomites from another source [29].
After physical treatment has a temperature of 1000 ° C for 2 hours, a change is observed in the phases; for crystalline forms of dolomite and calcite were thus disappeared quality diatomite became stronger due to the creation of new conditions after dissociation of calcium carbonate.
After chemical treatment with sulfuric acid, the X-ray diffraction shows the total change of the crystalline form of quartz which is mounted peaks quality diatomite more than physical treatment and makes the treatment more perform confirmed by a large change of the peak 2theta = 27 ° to a position 2 theta = 12 ° again prove by images of SEM.
Analysis by infrared spectroscopy
 |
Figure 5: Infrared spectroscopy of the acid treated diatomaceous earth (a) 800 ° C heat treated (b) heat treated 900 ° c (c), heat treated to 1000 ° C (d). Click here to View figure |
The IR spectrum of the acid treated diatomaceous earth has the same adsorption bands that gross diatomite but the bands of the organic material to 718 354 cm-1, 876 488 cm-1 and especially at 1433.89 cm-1 are absent confirms that the organic impurities have been removed after the chemical treatment .the existing only absorption bands correspond to bands of the symmetric and asymmetric vibration of the bond SiO2 471.51 cm-1 669 178 cm-1 and 1101.15 cm-1.
Diatomite calcined to 1000 ° C only shows the presence of bands characteristic vibrations of the Si-O bond of the SiO2 silica indicating that silica occupies the entire surface of diatomite.
OH- group characteristics disappear.
Therefore these spectra confirm that the physical and chemical activation of the crude product a diatomite hydroxylation and hydrophilicity decreasing diatomite.
Test photodegradation
Before assessing the photocatalytic activity of catalysts M1 and M2, an experiment was designed to measure the percentage of degradation or adsorption of MeO on catalysts prepared under the conditions of the dark and under UV-VIS radiation wavelength 365nm. For these experiments we took 250 ml of MeO solution at a concentration of 10 mg / l according to the protocol described previously.
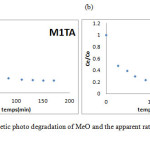 |
Figure 6(a), (b): Kinetic photo degradation of MeO and the apparent rate constant and M1TA M2TA Click here to View figure |
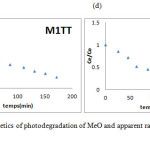 |
Figure 7 (c), (d): kinetics of photodegradation of MeO and apparent rate constant of M1TT and M2TT Click here to View figure |
According to the kinetic study, our hypothesis is that the phenomenon is mixed resulting in a rapid response after 30 minutes; by adsorption on the four MeO prepare materials followed by an oxidation reaction (photodegradation) of the dye in the presence of UV radiation.
Rate of photodegradation
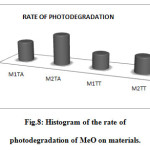 |
Figure 8: Histogram of the rate of photodegradation of MeO on materials. Click here to View figure |
From the results, the rate of photodegradation of M2TA material is higher by 84.22% contribution to other materials, and this because of the amount of diatomaceous earth used and made acid treatment.
Discussions
Figures 6 and 7 watches the results of the photocatalytic reaction and the effectiveness of catalysts prepared.
The following table shows the rate constants of the pseudo-first-order reaction and pseudo-second order of the four prepared materials
Table 3: pseudo-first-order reactions rate constants and pseudo-second order of the four materials
|
adsorbants |
1 er ordre |
2 eme ordre |
|
|
M1TA |
K=1,873 |
K=0,0192 |
|
|
R2 =0,9979 |
R2 =0,97 |
||
|
M1TT |
K=0,3717 |
K=0,0917 |
|
|
R2 =0,9945 |
R2 =0,96 |
||
|
M2TA |
K=0,6099 |
K=0,0018 |
|
|
R2 =0,940 |
R2 =0,085 |
||
|
M2TA |
K=0,9754 |
K=0,0128 |
|
|
R2 =0,949 |
R2 =0,74 |
||
Overall all catalysts showed good photocatalytic activity.
The experimental results showed a degradation of efficiency for the MeO and predominance for the second material.
Conclusion
The photocatalytic oxidation has proven to be a good process and promising to treat polluted solutions. Selecting a catalyst on an industrial scale of easier and less expensive treatment processes in operating conditions may be taken into account such as diatomite physically and chemically treated.
Performance and M1TA M1TT catalysts M2TA, M2TT prepared a basic diatomite and TiO2 in the form of nanoparticles was investigated by the photocatalytic oxidation of methyl orange. The degradation of the pollutant used as a model also shows a good photocatalytic activity for catalysts. An M2TA catalyst immobilization result seems to be the best by the other input, thanks to the large amount of diatomaceous earth which is greater than the amount used in the M1 catalysts. Diatomite due to its porosity and specific surface has low catalytic activity [30]. Previously, studies have shown that the Diatomite was a better adsorbent [24]. The kinetic study of the photodegradation follows a pseudo-first order model in our experiments.
Acknowledgements
We would also like to express our gratitude to the laboratory team LSTGP for their help and encouragement throughout completion of the article.Our sincere thanks also go to all our teachers and colleagues at Faculty of Chemistry (USTO -MB). Our wish also reflects our deepest recognition to those who encouraged us to finish this work by signs of friendship which we are grateful.
References:
- Bergamini, R.B.M., Azevedo, E.B., Araujo, L.R.R., Heterogeneous photocatalytic degradation of reactive dyes in aqueous TiO2 suspensions: decolorization kinetics. Chemical Engineering Journal 2009.49, 215–220.
- Han, F., Kambala, V.S.R., Srinivasan, M., Rajarathnam, D., Naidu, R. Tailored titanium dioxide photocatalysts for the degradation of organic dyes in wastewater: a review. Applied Catalysis A: General 2009 359, 25–40.
- Mahvi, A.H., Ghanbarian, M., Nasseni, S., Khairi, A. Mineralization and discoloration of textile wastewater by TiO2 nanoparticles. Desalination 2009 239, 309–316.
- Faisal, M., Abu Tariq, M., Muneer, M. Photocatalysed degradation of two selevted in UV-irradiated aqueous suspensions of titania. Dyes and Pigments 2007 72, 233–239.
- Saquib, M., Abu Tariq, M., Haque, M.M., Muneer, M, Photocatalytic degradation of disperse blue 1 using UV/TiO2/H2O2 process. Journal of Environmental Management 2008 88, 300–306.
- Singh, H.K., Saquib, M., Haque, M.M., Muneer, M.,. Heterogeneous photocatalysed decolorization of two selevted dye derivatives neutral red and toluidine blue in aqueous suspensions. Chemical Engineering Journal 2008 136, 77–81.
- Li, Y., Ma, M., Sun, S., Wang, X., Yan, W., Ouyang, Y.,. Preparation and photocatalytic activity of TiO2—carbon surface composites by supercritical pretreatment and sol–gel process. Catalysis Communications 2008 9, 1583–1587.
- Suwanchawalit, C., Wongnawa, S Influence of calcinations on the microstructures and photocatalytic activity of potassium oxalate-doped TiO2 powders. Applied Catalysis A: General 2008.338, 87–99.
- Wang, L., Yang, F., Ji, T., Yang,Q., Qi,X., Du,H., Sun, J.,. Preparation and characterization of Ti1−xZrxO2/ZrO2 nanocomposite. Scripta Materialia 2008 58, 794–797.
- Yang, X., Zhu, H., Liu, J., Gao, X., Martens, W.N., Frost, R.L., Shen, Y., Yuan, Z. A mesoporous structure for efficient photocatalysts: Anatase nanocrystals attached to leached clay layers. Microporous and Mesoporous Materials., 2008 112, 32–44.
- Valverde, J.L., Lucas, A., Sánchez, P., Dorado, F., Romero, A.,. Cation exchanged and impregnated Ti-pillared clays for selective catalytic reduction of NOx by propylene. Applied Catalysis B: Environmental 2003.,43, 43–56.
- Dékány, I., Pernyeszi, T.,. Photocatalytic degradation of hydrocarbons by bentonite and TiO2 in aqueous suspensions containing surfactants. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2004 230, 191–199.
- Dékány, I., et al. Photocatalytic oxidation of organic pollutants on titania–clay composites. Chemosphere 2008 70, 538–542.
- Rossetto, E.; Beraldin, R.; Penha, F. G.; Pergher, S. B. C. Caracterização de argilas bentonitas e diatomitas e sua aplicação como adsorventes. Química Nova 2009, 32, 2064.
- Anne danion, jean Didier, Chantal guillard. Fethi abdelmalk, Nicole raffrezie. Characterization and study of a single-TiO2-coated optical fiber reactor. Applied Catalysis B: Environmental 2004 52, 213.
- M. Karches, M. Morstein, P.R.v. Rohr, R.L. Pozzo, J.L. Giombi, M.A. Baltana´s, Plasma-CVD- coated glass beads as photocatalyst for water decontamination Catal. Today 2002 72 267.
- Lee, J. C., Kim, M. S. and Kim, B. W Removal of Paraquat Dissolvedin a Photoreactor with TiO2Immobilized on the Glass-Tube of UV Lamps Wat. Res2002. 36 1776
- S. Horikoshi, N.Watanabe, H. Onishi, H. Hidaka, N. Serpone, Photodecomposition of a nonylphenol polyethoxylate surfactant in a cylindrical photoreactor with TiO2 immobilized fiberglass cloth ,Appl. Catal.B 2002 37 117.
- I.N. Martyanov, K.J. Klabunde, Comparative study of TiO2 particles in powder form and as a thin nanostructured film on quartz, J. Catal 2004. 225 408–416
- J. Shang, W. Li, Y. Zhu, Structure and Photocatalytic Characteristics of TiO2 Film Photocatalyst coated on Stainless Steel Webnet, J. Mol. Catal. A 2003 202 187.
- H. Chen, S.W. Lee, T.H. Kim, B.Y. Hur, Photocatalytic decomposition of benzene with plasma sprayed TiO2-based coatings on foamed aluminum J. Eur. Ceram. Soc 2006. 26 2231
- C.H. Ao, S.C. Lee, J.C. Yu, Photocatalyst TiO2 supported on glass fiber for indoor air purification: effect of NO on the photodegradation of CO and NO2, J. Photochem. Photobiol. A 2003 156 171–177.
- M.S. Vohra, K. Tanaka, Photocatalytic degradation of aqueous pollutants using silica-modified TiO2 Wat Res. 2003 37 3992.
- A.H.C. Chan, J.F. Porter, J.P. Barford and C.K. Chan, Effect of thermal treatment on the photocatalytic activity of TiO2 coatings for photocatalytic oxidation of benzoic acid, J. Mater. Res. 2002, 17 1758–1765.
- PK Surolia, RJ Tayade, RV Jasra,TiO2-coated cenospheres as catalysts for photocatalytic degradation of methylene blue, p-nitroaniline, n-decane, and n-tridecane under solar irradiation Industrial & Engineering Chemistry Research 49 (19), 8908-8919
- R. J. Tayade, R. G. Kulkarni and R. V. Jasra, “Enhanced Photocatalytic Activity of TiO2-Coated NaY and HY Zeolites for the Degradation of Methylene Blue in Water,” Industrial and Engineering Chemistry Research, 2007 46 369-376.
- RJ Tayade, PK Surolia, MA Lazar, RV Jasra, Enhanced photocatalytic activity by silver metal ion exchanged NaY zeolite photocatalysts for the degradation of organic contaminants and dyes in aqueous medium Industrial & Engineering Chemistry Research 47 (20), 7545-7551
- TK Pathak, NH Vasoya, TS Natarajan, KB Modi, RJ Tayade, Photocatalytic degradation of aqueous nitrobenzene solution using nanocrystalline Mg-Mn ferrites , Materials Science Forum 764, 116-129
- Hosseini SN, Borghei SM, Vossoughi M, Taghavinia N.,. Immobilization of TiO2 on perlite granules for photocatalytic degradation of phenol. Applied Catalysis B: Environmental. 2007,74 ;53-62
- D. Vione, C. Minero, V. Maurino, M. E. Carlotti, T. Picatonotto, E. Pelizzetti,. Degradation of phenol and benzoic acid in the presence of a TiO2-based heterogeneous photocatalyst, ApplCatal B: Environ 2005.,, 58, 798

This work is licensed under a Creative Commons Attribution 4.0 International License.









