CuO Nanoparticles as an Efficient Catalyst for the Synthesis of Flavanones
Amanollah ZareiAhmady1*, Mosadegh Keshavarz2, Maryam Kardani1 and Neda Mohtasham3
1Department of Medicinal Chemistry, School of Pharmacy, Nanotechnology Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
2Department of Gas and Petroleum, Yasouj University, Gachsaran, Iran.
3Resident of Pediatrics, Abuzar Children`s Medical Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
Corresponding Author: E-mail address: zarei-a@ajums.ac.ir
DOI : http://dx.doi.org/10.13005/ojc/310369
Article Received on :
Article Accepted on :
Article Published : 31 Aug 2015
CuO nanoparticle was found to be an efficient catalyst for the synthesis of flavanones derivatives from the reactions between 2-hydroxyacetophenones and aromatic aldehydes at room temperature. The protocol offers advantages in terms of higher yields, short reaction times, and mild reaction conditions, with reusability of the catalyst. The structures of the synthesized substituted flavanones were confirmed by their Melting point, FT-IR, 1HNMR, 13CNMR and mass spectral analysis.
KEYWORDS:Flavanone; CuO; Nanoparticle; Solid Catalyst; Heterogeneous
Download this article as:| Copy the following to cite this article: ZareiAhmady A, Keshavarz M, Kardani M, Mohtasham N. CuO Nanoparticles as an Efficient Catalyst for the Synthesis of Flavanones. Orient J Chem 2015;31(3). |
| Copy the following to cite this URL: ZareiAhmady A, Keshavarz M, Kardani M, Mohtasham N. CuO Nanoparticles as an Efficient Catalyst for the Synthesis of Flavanones. Orient J Chem 2015;31(3). Available from: http://www.orientjchem.org/?p=10505 |
Introduction
Flavonones are known to exhibit numerous useful biological properties including antimicrobial, antitumor, antioxidant, and anti-inflammation.1-5 The methodology most widely used to prepare flavanones involves the isomerization of appropriately substituted 2-hydroxy chalcones, in turn achieved by an aldol condensation reaction between a 2-hydroxyacetophenone and an aldehyde. These cyclizations have been performed under numerous conditions using acids,6 bases,7 silica gel,8 heat,9 light,10 electrolysis,11 Ni/Zn/K halides12 and others.13 Other alternative processes to synthesize flavanones contain oxidation of flavan-4-ol.14 Reacting benzaldehydes with 1-(2-hydroxyphenyl)-3-phenyl propane- 1,3-diones in basic medium15 and transformation of 3-bromo-1-phenylprop-2-ynyl aryl ethers in the presence of mercury(II) trifluoroacetate.16
In recent years, nano-catalysis has emerged as a sustainable and inexpensive alternative to conventional catalysis since the nanoparticles possess a high surface-to-volume ratio, which improves their activity and selectivity, while at the same time preserving the essential features of a heterogeneous catalyst.17-20Nanocrystalline metal oxides find excellent applications as active adsorbents for gases and destruction of hazardous chemical.21They are also gaining tremendous importance due to their distinct catalytic activities for various organic transformations.
In continuation of our studies on nanotechnology17-20,22-24, herein, we wish to report a method for synthesis of flavanones at a strong basic media using CuO nanoparticles as an efficient nanocatalyst.
Experimental
Materials and methods
All chemicals and solvents were purchased from Sigma Aldrich. Merck silica gel F254 plates were used foranalytical TLC. Melting points were determined and analyzed in open capillary tubes in an Electrothermal IA 9100 melting point apparatus.The IR spectra were obtained on a Shimadzu 470 spectrophotometer (potassium bromide disks).The 1HNMR and 13CNMR spectra were recorded using Bruker 400 spectrometer. XRD (Philips PW 1800) and TEM (LEO 912AB) were used to characterize the copper oxide nanoparticles.
Preparation of CuO nanoparticle
For the synthesis of nano copper oxide,copper(II) nitrate trihydrate (Merck A G. For synthesis) was used as the precursor. Preparation of CuO took place in a stainless steel autoclave that was able to endure working temperature and pressure of 550oC and 610 at m, respectively. Concentration of Cu(NO3)2 was 0.05 mol dm-3, and heating period about 2 h. Synthesis was carried out at 500oCto accelerate the hydrolysis reactions. After removing from furnace, the autoclave was quenched by cold water and CuOnano particles were recovered from discharged solution by high speed centrifugation at 14,000 rpm for about 60 min. The produced nanoparticles were then three times washed in the same centrifuge with ultra-pure water, and then dried at ambient temperature.25
Synthesis of flavanones
General procedure for the synthesis of flavanone derivatives
A general procedure for the synthesis of flavanone derivatives was carried out by mixing of 1 mmol of 2′-hydroxyacetophenone and 1.4 mmol of aromatic aldehyde in 10 mL ethanol. CuO nanoparticle (5mmol%) was added and the reaction was stirred at room temperature and monitored by TLC. After completion of the reaction as indicated in table 1,the nanocatalyst was separated by high speed centrifugation. The product was precipitated by addition of the water drop by drop into the remaining solution.
Result and discussion
The XRD pattern of nano-sized is shown in Fig. 1. All diffraction peaks of X-ray are indexed to the monoclinic crystal system of CuO.No characteristic peaks are observed for other possible impurities,such as Cu(OH)2,Cu2O or Cu(OH)3NO3.
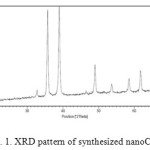 |
Figure 1: XRD pattern of synthesized nanoCuO Click here to View figure |
Average size of the obtained CuO particle shown in Fig. 2 is 10 nm. The crystallite size was also calculated by X-ray line broadening analysis using the Scherrer equation. It was found that the average CuO crystallite size is15 nm.The mean value of surface area of CuO catalyst was 37.58 m2/g from BET analysis.
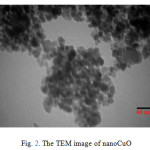 |
Figure 2: The TEM image of nanoCuO |
In an initial reaction, we tried to synthesize the flavanone starting with benzaldehyde and 2′-hydroxyacetophenone by using 1mmol% of CuOnano particles in ethanol in which the reaction was monitored by TLC. The isolated product was obtained in good yield (Scheme 1).
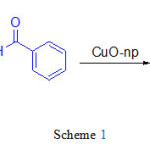 |
Scheme 1 Click here to View scheme |
In the absence of catalyst, the desired flavanone was obtained in trace yield in 8 h (Table 1).Increasing the loading of CuO nanoparticles to 5mmol% gave the title product in 87% yield in 2 h. Thusan increase in the concentration of catalyst not only promotesthe reaction but also resulted in an increased yield (Table 1).
Table 1: Optimization of the catalyst amount
|
Entry |
CuO-np (mmol%) |
Time (h) |
Yield(%) |
|
1 |
0 |
8 |
Trace |
|
2 |
1 |
6 |
45% |
|
3 |
2 |
4 |
70 |
|
4 |
5 |
2 |
87% |
In order to find the efficiency and the superiority of nanoCuO catalyst, we continued our study by comparing the catalytic activity of CuO nanoparticles with other traditional solid lewis base catalyst. The CuO nanoparticle catalyst was used along with other known solid bases as catalysts for the reaction between benzaldehyde and 2′-hydroxyacetophenone in 10 mL of ethanol as a model system for the synthesis of the corresponding flavanone derivative. The results are summarized in Table 2. Among all of the catalysts tested CuO nanoparticles proved to be the most efficient (Table 2).
Table 2: Synthesis of flavanone by using of different catalyst
|
Entry |
Catalyst(mmol%) |
Time (h) |
Yield(%) |
|
1 |
CuO-np (5%) |
2 |
87 |
|
2 |
Bulk CuO (5%) |
4 |
40% |
|
3 |
Al2O3 (5%) |
6 |
60 |
|
4 |
MgO (5%) |
4 |
78% |
The size of CuO plays an important role in terms of yields and reaction times. Changing the size of the particles from nanoparticles to bulk resulted in a drop in the catalytic activity. It is interesting to note that the CuO nanoparticle catalyst catalyzes the present reaction in high yield within a short reaction time compared to the other solid base catalysts.The increased catalytic activity of nanoCuO over commercially available bulk CuO may be attributed to the higher surface area of nano CuO than bulk CuO as well as the higher surface concentration of the reactive sites.
After obtaining best results by using of nanoCuO as catalyst for the preparation of flavanone derivatives, the reaction was extended to other substituted benzaldehyde and 2′-hydroxyacetophenone and the results are depicted in Table 3.
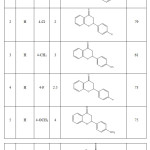 |
Table 3: Synthesis of flavanone derivatives by using of nanoCuO as efficient catalyst Click here to View table |
In a mechanistical point of view, it is envisioned that the reaction proceeds throughenolate intermediate at the first step followed by aldol-type condensation with bezaldehyde and finally an intramolecular cyclization leads to corresponding flavanone derivative (Scheme 2).
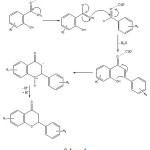 |
Scheme 2 Click here to View scheme |
CuO nanoparticle catalyst was found to be reusable, however gradual decline of activity was observed. Better results were obtained when, after the first run, the catalyst was separated by centrifugation, and the remained catalyst was washed with ether and dried at 150oC overnight, and reused. The reusability study applied for the reaction between benzaldehyde and 2′-hydroxyacetophenone as model reaction. Table 4 represents the comparison of efficiency of catalyst after five times.
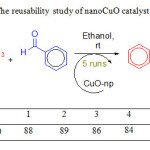 |
Table 4: The reusability study of nanoCuO catalyst Click here to View table |
Conclusion
In conclusion, CuO nanoparticle catalyst was simply prepared. The prepared heterogeneous catalysts were well characterized. The catalytic efficiency of the prepared catalysts was investigated for the preparation of flavanone derivatives. In the presence of the prepared solid catalyst, all the reactions were performed in short reaction times and gave the corresponding products at high yields. Nano sized particles,with a high surface‐to‐volume ratio, simple workup and recovery, and reusability up to five consecutive runs make this a new, efficient, and superior catalyst for the synthesis of flavanone derivatives.
Acknowledgement
This Research Project has been financially supported by Ahvaz Jundishapur University of Medical Sciences. (grant no. N60)
References
- Yanling, L., Hao, F., Wenfang, X., 2007. Mini Rev. Med. Chem. 7,663–678.
- Cushnie, T.P.T., Lamb, A.J., 2005. Int. J. Antimicrob. Agents 26, 343–356.
- Young, J., Park, Y., Lee, Y.U., Kim, H., Shim, Y.H., Ahn, J.H., Lim,Y., 2007. J. Microbiol. Biotechnol. 17, 530–533.
- Pan, M.H., Lai, C.S., Ho, C.T., 2010. Food Funct. 1, 15–31.
- H. Usman, E.H. Hakim, T. Harlim, M.N. Jalaluddin, Y.M. Syah, S.A. Achmad,H. Takayama, Z. Naturforsch. C 61 (2006) 184e188.
- Chaturvedi, R.; Patil, P. N.; Mulchandani,N. B. Indian J. Chem.1992, 31B, 340–341.
- Patonay, T.;Litkei, G.; Zsuga, M.; Kiss, A. Org. Prep. Proced. Int.1984, 16, 315–319
- Sangawan, N. K.; Varma, B. S.; Dhindsa, K. S. Chem. Ind.(London) 1984, 271–272.
- Hoshino, Y.; Takeno, N. Bull. Chem. Soc. Jpn. 1986, 59, 2903–2904.
- Maki, Y.; Shimada, K.;Sako, M.; Hirota, K. Tetrahedron1988, 44, 3187–3194.
- Sanicanin, Z.; Tabakovic, I. Tetrahedron Lett. 1986, 27,407–408.
- Ali, S. M.; Iqbal, J.; Ilyas, M. J. Chem. Res.1984,236–240.
- Dauzonne, D.; Grandjean, C. Synthesis1992,677–680.
- Sing, O. V. Org. Prep. Proced. Int.1993, 25, 693–695.
- Joglekar, S. J.; Samant, S. D. Tetrahedron Lett. 1988, 29,241–244.
- Subramanian, R. S.; Balasubramanian, K. K. J. Chem. Soc., Chem. Commun.1990, 1469–1470.
- Albadi, J.; Keshavarz, M.; Shirini, F. andVafaie-nezhad, M. Catal. Commun.,2012,27, 17.
- Albadi, J.; Keshavarz, M.; Abedini, M. andVafaie-nezhad, M. Chin. Chem. Lett., 2012,23, 797.
- Keshavarz, M.; Iravani, N.; Ghaedi,A.; ZareiAhmadi, A.; Vafaie-nezhad, M.; Karimi, S.SpringrPlus, 2013, 2, 64.
- Iravani, N.; Keshavarz, M.; Shojaeian Kish, A.; Parandvar, R. Chine. J. Catal.2015, 36, 626.\
- Astruc, D.; Nanoparticles and Catalysis, Wiley-VCH, Weinheim, 2008.
- Heidarizadeh, F.;Zarei A.Asian J. Chem., 2008, 20, 1514.
- Fadavipoor, E.; Nazari, S.; ZareiAhmadi, A.;Gorjizadeh, M.; Afshari, M.; Keshavarz, M. Orient. J. Chem., 2015, 31, 733.
- Saadat, S.; Nazari, S.; Afshari, M.; Shahabi, M.; Keshavarz, M. Orient. J. Chem., 2015, 31, 1005.
- Ahmadi, S. J.; Sajadi, S.; Hosseinpour, H.; Outokesh, M.; Hekmatshoar, R. Catal.Commun.2009, 10, 1423.

This work is licensed under a Creative Commons Attribution 4.0 International License.









