Combined Electrochemical Method of Synthesis of Lead Tetraacetate From Metallic Lead
M. S. Satayev1, A. P. Aueshov2, Sh. T. Koshkarbayeva1, L. M. Satayeva3, and A. S. Tukibayeva4*
1Department of Chemical technology of inorganic substances, M.Auezov South Kazakhstan State University, South-Kazakhstan region, Republic of Kazakhstan.
2Laboratory of physico-chemical methods of researches, M.Auezov South Kazakhstan State University, South-Kazakhstan region, Republic of Kazakhstan.
3Department of Ecology, M.Auezov South Kazakhstan State University, South-Kazakhstan region, Republic of Kazakhstan.
4Department of Chemistry, M.Auezov South Kazakhstan State University, South-Kazakhstan region, Republic of Kazakhstan M.Auezov South Kazakhstan State University, South-Kazakhstan region, Republic of Kazakhstan.
Correspodence Author Email: ainur_ tukibaeva@mail.ru
DOI : http://dx.doi.org/10.13005/ojc/310311
Article Received on :
Article Accepted on :
Article Published : 29 Jul 2015
Electrochemical synthesis of lead tetraacetate using a flow diaphragm and using as the initial reagent instead of metallic lead was studied. It is shown that the creation of the catholyte flow into the anolyte through the diaphragm significantly reduces a cell voltage. The possibility of additional saturation of anolyte with diacetate of lead at the expense of chemical dissolution of metallic lead was identified. This allows to eliminate the use of lead diacetate trihydrate from the process flow sheet, the use of which requires a dehydration operation by expensive acetic anhydride. These additions simplify the technology of electrosynthesis and economic indexes of process improve.
KEYWORDS:lead tetraacetate; lead; electrosynthesis; flow diaphragm
Download this article as:| Copy the following to cite this article: Satayev M. S, Aueshov A. P, Koshkarbayeva Sh. T, Satayeva L. M, Tukibayeva A. S. Combined Electrochemical Method of Synthesis of Lead Tetraacetate From Metallic Lead. Orient J Chem 2015;31(3). |
| Copy the following to cite this URL: Satayev M. S, Aueshov A. P, Koshkarbayeva Sh. T, Satayeva L. M, Tukibayeva A. S. Combined Electrochemical Method of Synthesis of Lead Tetraacetate From Metallic Lead. Orient J Chem 2015;31(3). Available from: http://www.orientjchem.org/?p=10090 |
Introduction
Lead tetraacetate (LTA) is a selective oxidant, and is widely used in organic chemistry in various preparative synthesis1-3. There are scientific works4-5 on the investigation the effect of temperature and pH in adsorption of Pb+2 ions by porous Perlite clay and studies on the analysis of lead and Silica in lead processing samples.
It is usually prepared by dissolving lead oxide in anhydrous acetic acid, heated until 55-65°C1.
Рb3О4 +8СН3СООН → Рb(СН3СОО)4 + 2Рb(СН3СОО)2 + 4Н2О (1)
Formed by the reaction, water causes the hydrolysis of lead tetraacetate with the formation of dioxide.
Рb(СН3СОО)4 + 2Н2О → РbО2 + 2 СН3СООН (2)
Therefore, for binding of water acetic anhydride is added into the reaction medium. The harmful effect of water is also removed by lowering the temperature of carrying out synthesis6. Since interaction of red lead with anhydrous acetic acid at temperatures 30-40 °C with subsequent isolation of the desired product is not later than 3-5 hours after the ending of reaction provides lead tetraacetate yield of 70-75% of the theoretical. In this case, lead diacetate is a dehydrating agent, forming hydrated complex with water.
Equation 1 shows that only one atom of three atoms of lead in the composition of red lead participates in the formation of lead tetraacetate. Furthermore, when using lead tetraacetate in organic synthesis lead diacetate is usually a by-product2,3. Therefore, it is desirable re-use this product for the synthesis of lead tetraacetate that allows to propose a closed production cycle. However, in given method of synthesis of lead tetraacetate is not possible.
For obtaining lead tetraacetate has also been proposed an electrochemical method, which allows fuller use the feedstock and obtain a product of high purity7,8. The process is carried out in a medium of anhydrous acetic acid with the separation of the anode and cathode spaces by means of a ceramic diaphragm. The solution of lead diacetate and potassium acetate of conductive additive is an anolyte, potassium acetate solution is a catholyte. Platinum, lead dioxide and graphite are used as anodes8. The chemical resistance of anodes of lead dioxide during electrosynthesis of lead tetraacetate and ways of its increase are listed in9.
On the anode the electrochemical oxidation of diacetate lead into lead tetraacetate occurs.
Рb(СН3СОО)2 + 2СН3СООН –2е → Рb(СН3СОО)4 + 2Н+ (3)
On the nickel cathode hydrogen is released:
2Н+ + 2 е → Н2 (4)
The overall reaction in electrolytic cell is:
Рb(СН3СОО)2 + 2СН3СООН → Рb(СН3СОО)4 + Н2 (5)
In order to isolate lead tetraacetate, formed during electrolysis, an anolyte is output from electrolytic cell and is cooled. At this the precipitated crystals are separated by filtration, and the anolyte is saturated with anhydrous diacetate of lead and recycled to the electrolysis. The current efficiency is 80-90%
The electric cell voltage is 60-70 V at current density of 4 A/dm2.
Electrochemical method of synthesis of lead tetraacetate allows to use lead diacetate, obtained as a by-product in organic synthesis reactions involving lead tetraacetate. One of the variants of such technology is carrying out electrosynthesis of lead tetraacetate in a single cell and reactions of organic synthesis10. At this, acetoxylation of acetophenone and oxidation of styrene were conducted with yield of 68% and 35.6% respectively.
However, the wide application of electrochemical method of obtaining lead tetraacetate is prevented by a number of disadvantages. This is a high voltage and its increase during the electrolysis process and in connection with this the difficulties of maintaining the thermal regime of the electrolytic cell arise, as well as the necessity for additional saturation of anolyte with dehydrated lead diacetate.
The cell voltage depends on the value of distance between electrodes, the thickness and porosity of the ceramic diaphragm, potassium acetate content in electrolyte. In the given system potassium acetate is a main current carrier (acetic acid because of the weak dissociation and lead acetate due to the formation of anionic complexes practically doesn’t participate in the transfer of current) and at this electromigration of acetate ions from anolyte into catholyte is observed and vice versa potassium ions from anolyte into catholyte. When electricity is passed through the cell per 1 hour∙A from anolyte into catholyte 3.6 g of potassium acetate is transferred according to Faraday’s law. Because of this, the concentration of potassium acetate decreases in anolyte and increases in catholyte (crystals are even precipitated in catholyte volume). When using uncirculating diaphragm, it leads to a quite rapid increase of resistance of electrolyte.
Additional saturation of anolyte by dehydrated lead diacetate also has certain difficulties. Upon heating of typically used trihydrate Pb(CH3COO)2 ∙ 3H2O reagent along with removing water of crystallization, lead diacetate decomposes. It compels to conduct prolonged boiling of trihydrate with acetic anhydride.
The aim of this work is a creation of an alternative technology of electrochemical synthesis of lead tetraacetate, which the above-mentioned disadvantages would be eliminated. For this processes with the flow of catholyte through the diaphragm into anolyte and additional saturation of anolyte in respect to diacetate by dissolving of metallic lead were investigated.
Material and Methods
Laboratory investigations were carried out in an electrolytic cell of the following design (Fig. 1).
An electrolytic cell body 1 is a glass vessel, equipped with a water jacket for maintaining the necessary temperature. The inner diameter of the vessel is 12 cm with working height of 10 cm. In the side wall of the vessel a tube 2 is welded for outputting an anolyte during circulation. The cover of the vessel 3, made of light rubber had holes for fixing diaphragm 4, carbon anodes 5, for supplying circulating anolyte 6 and backfill of granules of metallic lead 7. A thin-walled (1.2 mm) ceramic glass with an internal diameter of 3.5 cm is used as a diaphragm. The anodes of 4 mm thickness are cut out from a graphite tube with a diameter of 4.5 cm. At this graphite anodes and current lead were an integral, which allows realize an electrical contact with the current source outside the electrolytic cell. The diaphragm also has a cover 8, on which cathode 9 of nickel wire, twisted into spiral is fastened. The cover also has holes for output tube, released hydrogen 10 on the cathode and a filling of consumed acetic acid 11 during electrolysis. Theoretical consumption of acetic acid on electrolysis is small about 2.5 ml per 1 A ∙ hour or 38 ml per 1 hour at a current load of 15 A. But if we take into account the carry-over by gaseous hydrogen and the loss due to evaporation of an electrolyte, this consumption increases by more than 2 times. Refill water of electrolyte by acetic acid is advantageous to carry out through catholyte, at this a useful dilution of catholyte by potassium acetate happens. In order to facilitate overflow of portion of catholyte into anolyte it is necessary to maintain a higher level of catholyte as compared with anolyte. In this cell design exceed of catholyte level over anolyte is 3 cm. If the overflow of catholyte due to the porosity of diaphragm does not create a sufficient change of concentration of potassium acetate in anolyte, an additional overflow can occur through the opening 12 in diaphragm. The rate of overflow was regulated by a tap when filing acetic acid through a tube 11. When a sufficient overflow rate of catholyte into anolyte an electrical voltage on electrolytic cell does not change. Therefore, overflow rate was regulated based on this parameter.
Lead granules 13, loaded into anolyte, were placed in lower part of electrolytic cell and did not in contact with anode.
Based on the research8 were carried out at the temperature of 80 °C. At this the initial concentrations are: anolyte Pb(CH3COO)2 -2 eq/L, CH3COOK -1 g-eq/L and catholyte CH3COOK – 1 g-eq/L.
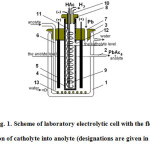 |
Figure1: Scheme of laboratory electrolytic cell with the flow of portion of catholyte into anolyte (designations are given in the text). Click here to View figure |
Laboratory electrolytic cell provides a stable work when an ampere loads of up to 15 A. At higher currents, there are difficulties with the maintenance of temperature regime of electrolysis. Therefore, during investigations for different current densities the surface of anodes were chosen so that the amperage during electrolysis does not exceed 15 A.
For obtaining lead tetraacetate the possibility of using metallic lead for additional saturation of anolyte with lead diacetate was also studied. Upon dissolution of metallic lead in dehydrated acetic acid, anhydrous salt of lead is directly obtained. Thus, operations of dehydration of lead diacetate are eliminated, which simplifies the process flowsheet. In such a combined scheme is easier to maintain the necessary concentration of lead diacetate. If when additional saturation of anolyte with concentrated solutions of Pb(CH3COO)2 there is some dilution by potassium acetate8, it does not occur in the proposed method.
It is possible to dissolve lead in this given medium, using an additional electrolytic cell with lead anode, but this complicates the technological process, so a chemical dissolution of lead is more preferable due to its oxidation, formed during electrolysis of lead tetraacetate. If half of obtained product during electrolysis of lead tetraacetate will spend for chemical dissolution of metallic lead, lead diacetate content in anolyte changes little even after prolonged electrolysis.
Our researches have shown that this reaction proceeds quantitatively independently of concentration of lead diacetate and potassium acetate. At this the rate of dissolution of lead is much higher than the rate of oxidation diacetate into tetraacetate. Thus, 500 ml of a solution, containing 400 g/L of Pb(CH3COO)2, 100g/l of CH3COOK, 100 g/L of lead tetraacetate at 80 °C lead plate, having a surface of 0.1 dm2 lost 5 g in weight for 8 minutes, which corresponds to a current density of dissolution of 96 A/dm2. Therefore, up to this current density a stage of chemical dissolution of lead can not be limiting for the overall rate of process of obtaining the lead tetraacetate.
Laboratory investigations were carried out under conditions, ensuring the flow of catholyte through diaphragm, and the data of electrolysis without flow of catholyte are presented for comparison. Duration of electrolysis with flow was 5 hours. At this anolyte is also subjected to forced circulation through the outer vessel with the speed 0.5 liters/hour. Loading granules of metallic lead into electrolytic cell were produced based on the calculation
m=0,5τqη
where m is a mass of loaded lead; 0.5 is a load factor, which provides complete regeneration of spent lead diacetate for electrolysis; τ is a duration of electrolysis for which it is necessary to compensate the spent lead diacetate by electrolysis (in our experiments is 0.5 hours); q is an electrochemical equivalent of lead, 3.86 g / (A ∙ h); η is a current efficiency of lead tetraacetate, 0.8-0.9.
Lead tetraacetate was isolated both in an electrolytic cell and in the outer vessel.
For electrolysis without flow of catholyte the duration of electrolysis was 1 hour and circulation of anolyte was not carried out.
The formed crystals of lead tetraacetate were collected by filtration on a paper filter, washed with acetic acid and dried in a desiccator over sulphuric acid until constant weight. The current efficiency of lead tetraacetate was calculated based on the equation of 8.
Results and Discussions
During electrolysis with the addition of metallic lead into an anolyte in anode space of the electrolytic cell the following reactions occur:
On graphite anode
2Рb(СН3СОО)2 + 4СН3СОО–– 4е→2Рb(СН3СОО)4 (6)
In volume of anolite
Рb(СН3СОО)4 + Рb→2Рb(СН3СОО)2 (7)
The overall reaction in anode space
Рb + 4СН3СОО–– 4е→Рb(СН3СОО)4 (8)
Taking into account the cathodic reaction of hydrogen release the total reaction in an electrolytic cell will be in the form
Рb + 4СН3СООН→Рb(СН3СОО)4 + 2Н2 (9)
Table 1 shows the results of electrolysis with flow and without flow of catholyte through diaphragm. The data in Table shows that when flowing catholyte a significant reduction of voltage in cell was achieved. In addition, the voltage reduction, improving the thermal regime of cell, allows to increase a limit of current density, at which the high current efficiency remains. When conducting synthesis with a forced circulation of anolyte and with an external heat removal, an optimal current density may be increased up to 50 A/dm2.
Table1: Comparative characteristics of the process of obtaining lead tetraacetate in electrolytic cell with flowing and uncirculating diaphragm. Anode is graphite. Anolyte: Pb(CH3COO)2 – 2 n, CH3COOK – 0.6n.
| № |
Type of electrolytic cell |
Anode current density, A/dm2 |
Cell Voltage, V |
Current efficiency of lead tetraacetate formation, % |
| 1 | With flow diaphragm | 7,5 | 15 | 90 |
| 2 | 15 | 28 | 90 | |
| 3 | 25 | 32 | 85 | |
| 4 | 50 | 45 | 72 | |
| 5 | With uncirculating diaphragm | 7,5 | 25-27 | 90 |
| 6 | 15 | 36-38 | 84 | |
| 7 | 25 | 48-53 | 72 | |
| 8 | 50 | 60-65 | 60 |
Combination of processes for obtaining bivalent lead from metallic lead and its further electrochemical oxidation into lead tetraacetate not only simplifies the technology for producing lead tetraacetate, and the economic indexes are also improved (Table 2). Thus, in comparison with the conventional method8 replacement of expensive lead diacetate and acetic anhydride leads to a significant reduction in raw material costs.
Despite the fact that the half of formed lead tetraacetate by electrolysis is spent on chemical oxidation of lead into lead diacetate (actual electrochemical equivalent of lead tetraacetate is halved), a significant increase of power consumption doesn’t occur. It is connected with the use of flowing diaphragm, contributing to significantly reducing the voltage in an electrolytic cell.
Table2: Comparison of indicators under expenses on reagents and electricity in conventional and combined electrolysis (prices are as of February 2015).
| № |
Reagent or |
Consumption per 1 kg of lead tetraacetate |
|||
|
In material expression, kg |
In price expression, $ |
||||
|
Conventional electrolysis |
Combined electrolysis |
Conventional electrolysis |
Combined electrolysis |
||
| 1 | Рb(СН3СОО)2∙3Н2О | 0,852 | – | 7,57 | – |
| 2 | (СН3СО)2О | 0,230 | – | 11,5 | – |
| 3 | СН3СООН | 0,270 | 0,540 | 0,742 | 1,484 |
| 4 | Pb in granules | – | 0,467 | – | 1,984 |
| 5 | Electric energy (only for electrolysis) | 8,070kW ∙ hour | 8,608kW ∙ hour | 0,7263 | 0,775 |
| Total | 20,538 | 4,243 | |||
Conclusion
The flow of catholyte into anolyte through diaphragm allows to maintain a certain concentration of potassium acetate in both spaces throughout electrolysis. This reduces the cell voltage.
Combination of process of obtaining lead tetraacetate with chemical dissolution process of lead allows to exclude a difficult implementing stage from technology, such as a dehydration of raw materials, and to replace an expensive reagents of lead diacetate and acetic anhydride by a relatively cheap metallic lead, which creates a favorable mode for prolonged electrolysis.
References
- Mijs W.J., De Longe, Comelis R.H.I., Organic Synthesis by Oxidation with Metal Compounds, Plenum. New York, (1986)
- Nikishin, G.I.; Sokova, L.L.; Makhayev, V.D.; Kapustin, N.I. Proceedings of Academy of Sciences, Series of chem., 2005, 973-975.
- Alvarez-Manzaneda, E.J.; Chahboun, R.; Alvarez, E.; Alvarez-Manzaneda, R.; Muñoz, P.E.; Jiménez, F.; Bouanou, H. Tetrahedron Letters, 2011, 52, 4017-4020
- Aminifard, S.; Jamalzadeh, H.; Biazar, E.; Fouladi, M. Orien. J.Chem, 2011, 27(4), 1397-1401
- Ravichandra Babu, R.; SAIKRISHNA, Y.V.S. Orien. J.Chem., 2013, 29(4), 1703-1706
- Russia Patent No 2277530, Tsinovoi, Yu.N.; Novotorov Yu. N.; Feshenko Hil. A., A method for obtaining lead tetraacetate, 10.06.2006.
- Jafarov E.A., Electrodeposition, properties and applications of lead dioxide, USSR Academy of Sciences. Baku, (1967)
- Satayev, M.S.; Bahchisaraytsyan, N.G.; Fioshin, M.Ya.; Kryschenko, K.I. Chem. Ind. 1970, 2, 852-854
- Pat. SU 130038. A method for reducing the porosity of anodes of lead dioxide
- YE Xiao-He,WANG Huan,XUE Teng. Chin. J. Org. Chem., 2007, 27(05), 643-647

This work is licensed under a Creative Commons Attribution 4.0 International License.









