Chemical Modification Methods of Nanoparticles of Silicon Carbide Surface
Anton S. Yegorov*, Vitaly S. Ivanov, Alexey V. Antipov, Alyona I. Wozniak, Kseniia V. Tcarkova.
Federal State Unitary Enterprise «State Scientific Research Institute of Chemical Reagents and High Purity Chemical Substances» (FSUE «IREA») 107076, Russia, Moscow, Bogorodskyval 3,.
Correspondence Author E-mail: egorov@irea.org.ru
DOI : http://dx.doi.org/10.13005/ojc/310303
Article Received on :
Article Accepted on :
Article Published : 22 Aug 2015
silicon carbide exhibits exceptional properties: high durability, high thermal conductivity, good heat resistance, low thermal expansion factor and chemical inactivity. Reinforcement with silicon carbide nanoparticles increases polymer’s tensile strength and thermal stability.Chemical methods of modification of the silicon carbide surface by means of variety of reagents from ordinary molecules to macromolecular polymers are reviewed in the review.The structure of silicon carbide surface layer and the nature of modificator bonding with the surface of SiC particles are reviewed. General examples of surface modification methodologies and composite materials with the addition of modified SiC are given.
KEYWORDS:carbide; nanostructures; nanopowder; modificator; surface modification; composite; composite material
Download this article as:| Copy the following to cite this article: Yegorov A. S, Ivanov V. S, Antipov A. V, Wozniak A. I,Tcarkova K. V. Chemical Modification Methods of Nanoparticles of Silicon Carbide Surface. Orient J Chem 2015;31(3). |
| Copy the following to cite this URL: Yegorov A. S, Ivanov V. S, Antipov A. V, Wozniak A. I,Tcarkova K. V. Chemical Modification Methods of Nanoparticles of Silicon Carbide Surface. Orient J Chem 2015;31(3). Available from: http://www.orientjchem.org/?p=10418 |
Introduction
Nanostructures represent a new class of materials that have at least one dimensionless than 100 nm. Nanostructures can be divided into 4 groups: zero-dimensional (0D), one-dimensional (1D), two-dimensional (2D) and three-dimensional (3D) structures. 0D structures are structures with nanoscale dimensions such as quantum dots (QD), nanoparticles and nanospheres; 1D nanostructures are structures with two nanoscale dimensions such as nanowires, nanotubes and nanoribbons; 2D structures have only one nanoscale dimension that as a rule is perpendicular to plane layer, for example,nanoplates, nanofilms and supperlattices; 3D nanostructures which are formed by creating an ensemble of nanostructures such as nanowires and nanorods. A large number of nanomaterials of all types of structures were explored. They are gradually being implemented into industry and everyday life [1], [2].
Being one of the most important compounds – semiconductor, silicon carbide exhibits exceptional properties such as wide (varied) range of bandgap, high durability, high thermal conductivity and heat resistance, low thermal expansion factor and chemical inactivity[3-5].
By reason of small dimensions, quantum and shape effects materials with nanostructured silicon carbide expectedly exhibit qualities that their bulk analogues might not have. Intensive studies were carried out to prepare SiC-nanostructures and to correlate their bonds and morphology with mechanical, optical and electrical properties [7-11].
Due to its outstanding properties such as high durability, high heat resistance and chemical inactivity SiC-nanomaterials are reinforcing extenders in polymer-matrix composites [12-14].
One of the main problems of nanocomposites obtaining is prevention of particle aggregation. It is rather difficult to obtain monodisperse particle distribution within the polymer matrix. This problem can be solved by modifying nanoparticles surface that improves interaction of inorganic modificator and polymer.
General information about SiC surface.
The nanoparticles surface layer composition and structure is critical in the interaction with the polymer. A lot of scientific works devoted to analysis of the surface layer of different material as well as purposeful change of its structure and properties are known. Depending on the pre-treatment the surface moisture and content of various adsorbents on it may vary. If necessary, various organic substituents or atoms/ions of some elements can be applied on the surface to give it specific properties (hydrophilicity/hydrophobicity or affinity to certain substances)
According to thermodynamic data we can say that silicon carbide is not an inert and stable to oxidation compound. For example Gibbs free energy change in the interaction with oxygen at 298K –strongly negative value – suggests the possibility of oxidation reaction at room temperature:
SiC(s) + 2/3O2(g) = SiO2(s) + CO(g) ΔG = –925 kJ/mole
However, it is known that perceptible interaction only occurs at temperatures above 1000° С. Moreover, silicon carbide doesn’t interact with such reagents as chlorine (below 600° C), bromine (below 800° C), sulfur (vapour) (below 900° C), fluorine (below 300° C) [15].
This is due to the presence of amorphous silica protective film on the SiC surface which is known to have extremely low diffusion coefficient for many gaseous substances in both molecular and atomic state [16]. The presence of protective surface layer plays a key role in silicon carbide chemistry. That’s why unmodified silicon carbide nanopowder surface and its behavior in colloidal solutions should be similar to the surface properties of colloidal silica. At the same time, colloidal silica is a rather explored object and lots of detailed works are devoted to it [17].
Modification Methods of SiC Surface
Two main modification methods are distinguished. The first one is realized by adsorption or interaction of surface layer with small molecules (silane, for instance). The second one is based on molecules grafting via covalent bonds to hydroxyl groups on the nanoparticles surface. The second method has an advantage since it allows to obtain particles with necessary and predictable properties due to fine particles selection possibility, grafting monomer and process conditions.
Chemical Treatment with Low-Molecular Agents
Nanoparticles surface modification by chemical treatment is a good method of improving nanoparticle dispersions stability in various fluids. The idea of silane using was proposed by Plueddemann and his colleagues [18]. Nanoparticles surface modification scheme is shown in the picture 1. The surface of unmodified nanoparticle is covered only with hydroxyl groups (-OH) while after modifying the surfaceis covered with residues of 3-methacryloxypropyltrimethoxysilane. Modified nanoparticles in organic solvents and in polymeric matrices behave differently than unmodified ones. For instance, modified nanoparticles exhibit improved dispersibility and stability of dispersions [19].
![Picture 1 – Surface modification by 3-methacryloxypropyltrimethoxysilane [19]. A wide range of compounds, for example silicon and metal alkoxides, epoxides, alkyl- and aryl-isocyanates etc. is used for modification. The presence of a large number of commercially available silaneswith various organic residues is of a special note[20].](http://www.orientjchem.org/wp-content/uploads/2015/08/Vol31_No3_Chem_Anto_Fig1-150x150.jpg) |
Figure 1: Surface modification by 3 methacryloxypropyltrimethoxysilane [19]. A wide range of compounds, for example silicon and metal alkoxides, epoxides, alkyl- and aryl-isocyanates etc. is used for modification. The presence of a large number of commercially available silanes with various organic residues is of a special note[20]. Click here to View figure |
Adsorption of Polymeric Dispersants on the Nanoparticles Surface
Modification by polymeric dispersants adsorption is one of the simplest ways to improve nanoparticles stability in aqueous systems. Hydrophilic nanoparticles can be dispersed in highly polar solvents using anionic or cationic polymeric dispersants. Such dispersants create volume repulsive forces between polymeric chains and increase surface charge that leads to improved dispersions stability. Polycarboxylic acids and their salts can be used as such dispersants [20].
Synthetic Polymers Grafting (Elongation)
This approach allows chemical and physical properties of both organic and inorganic polymers to be changed more efficient as well as surface topology changing. Such inorganic particles with grafted polymeric chains are considered organic-inorganic composites.
Since monomers usually have low molecular weight they can penetrate nanoparticles aggregates and interact with active sites on their surface. Nanoparticles aggregates inner space gets partially filled with macromolecular chains. After that aggregated nanoparticles can be separated easily [21]. By the way, this nanoparticles surface gets hydrophobic that is good for filler and matrix miscibility (picture 2).
![Picture 2 –Diagrammatic representation of agglomerated nanoparticles in polymeric matrix without polymer grafting (left) and divided by polymer elongation particles (right) [21].](http://www.orientjchem.org/wp-content/uploads/2015/08/Vol31_No3_Chem_Anto_Fig2-150x150.jpg) |
Figure 2: Diagrammatic representation of agglomerated nanoparticles in polymeric matrix without polymer grafting (left) and divided by polymer elongation particles (right) [21]. |
For polymers elongation two options of such composite obtaining are used. First one is referred to in literature as “grafting (elongation) on” wherein polymers with suitable terminal functional groups interact with corresponding surface. The second method is called “grafting (elongation) from” wherein polymeric chains grow from the monolayer of organic radicals ending with a fragment initiator of polymerization [22-24].
Examples of Silicon Carbide Surface Modification
There are relatively few publications devoted to silicon carbide powder surface modification (in comparison with silica and carbon nanotubes modification). All the works found are individual original research; reviews, monographs and books on this subject are not found. This is probably due to the fact that silicon carbide is used as a polymeric filler relativelyseldom but at the same time exhibits surface properties similar to silicas. In most studies authors use various commercially available silane agents for modification.
For example Bazzar and his colleagues [25] have obtained poly(triazole-imide)/SiC – nanocomposite. Authors are describing nanocomposites synthesis based on polyimide with pure SiC nanoparticles as well as with – glycidoxypropyltriethoxysilane (GPTES) modified SiC nanoparticles. Modified nano-SiC was stored in vacuum for moisture protection.
Original and modified nano-SiC analysis was carried out using Fourier IR-spectroscopy and DTA/DSC – analysis.
Diagrammatic representation of modified SiC surface is shown in the picture 3.
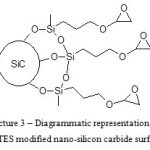 |
Figure 3: Diagrammatic representation of GPTES modified nano-silicon carbide surface. Click here to View figure |
The composite was obtained by reaction of diamine with dianhydride in the presence of SiC-nanoparticles followed by polyamide acid formation which was then heated in vacuum to give poly(triazole-imide)/SiC – nanocomposite. Filled nanocomposite breaking strength has increased to 156 MPa (unfilled – 108 MPa). Composite showed better thermal stability (5% of weight loss with temperature increase from 380 to 500° C).
Analogic compound of – glycidoxypropyltrimethoxysilane (referred to as KH-560) was applied on the silicon carbide micron filler surface [26]. They also explored obtainment of modified silicon carbide composites with epoxy which show improved mechanical strength. The authors found that in composite sample obtained covalent bond between the polymer and the organic modifier applied on the particles surface was formed. Methodology relating to surface modification is almost identical to the procedure described in previous work. Analysis methods used are the same.
In articles[27] and [28] polypyrrole/SiC-nanocomposites with various percentages of silicon carbide particles was being obtained by oxidative polymerization method. Ammonium persulfate and ferric chloride (III) act as an oxidant. This method can be regarded as a surface modification of the conductive polymer.
Silicon carbide micron fraction surface modification by aminosilanes is shown in article[29]. The following aminosilanes are used:
WD-50: (CH3CH2O)3SiCH2CH2CH2NH2,
WD-52: (CH3CH2O)3SiCH2CH2CH2NHCH2NH2,
WD-57: (CH3CH2O)2CH3SiCH2CH2CH2NH2.
This modification confered resistance silicon carbide aqueous suspensions and changed sol isoelectric point from pH=4,7 to 10.
Authors [30] modified the surface by azo-radicals using the following sources: 2,2′-azobisisobutyronitrile(AIBN), 2,2′-azobis(2-methylpropionamidine)dihydrochloride (AMPA), and 2,2′-azobis[N-(2-carboxyethyl)-2-methylpropionamidine)n-hydrate (ACMPA) (picture 4)
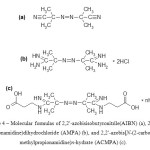 |
Figure 4: Molecular formulas of 2,2′-azobisisobutyronitrile(AIBN) (a), 2,2′-azobis(2-methylpropionamidine)dihydrochloride (AMPA) (b), and 2,2′-azobis[N-(2-carboxyethyl)-2-methylpropionamidine)n-hydrate (ACMPA) (c). Click here to View figure |
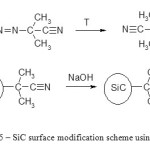 |
Figure 5: SiC surface modification scheme using AIBN |
Modified silicon carbide nanoparticles stable in aqueous solutions with different pH were obtained this way. In case of aemulsion they are stable at pH>5, b emulsion – at pH<3 and in case of c – stable at pH<3 and >11.
Rosso and coll.[31]were modified SiCsurface by covalently bonded alkens ending with a double bond. Samples are stable in strong acids solutions for 4 hours at 90°C and in alkaline solutions at pH=11.
A method of controlling the nano-SiC behavior in aqueous colloidal solution by applying a layer of aluminum oxide-hydroxide is known [32].Urea is used as a precipitating agent. Aluminum nitrate is used as a source of ions.
The amount of aluminum was taken so as to obtain a continuous SiC nanoparticles coating by aluminum oxide-hydroxide 1 nm thick assuming that all the aluminum was deposited.
The oxygen content in powders was determined by IR spectra of the gaseous products of heating the samples (Elta, Germany). Energy-dispersive X-ray spectrometer and X-ray photoelectron spectrometer (XPS) were used for chemical analysis. A transmission electron microscope was also used.
While exploring original powders authors found a silica layer 1-5nm thickas well as an oxycarbide intermediate layer on the particles surface.
After applying a layer of aluminum oxide-hydroxide powders changed behavior in a colloidal solution, becoming similar to the pure alumina colloid.
In article [33] Silicon carbide suspension was mixed with aluminum nitrate at a ratio of 4:3. Hexamethylenetetramine was used as a precipitating agent. The rheological properties of the particles and the surface charge were studied.
The authors [34] used a similar method of silicon carbide surface modification. They investigated the dependence of the aluminum oxide-hydroxide layer thickness as well as the surface charge depending upon the initial concentration of the aluminum ions source (aluminum nitrate) and the pH of the environment. Then they modified the surface by two polyelectrolytes Dispex A40 and KA11 thushaving obtained SiC/Al2O3 particles stable in water.
A similar method is proposed in the article [35]. Aluminum triisopropoxide is used as a source of aluminum and as a solvent for the process – pre-dried n-hexane. Results and conclusions are generally similar to the previous work.
Conclusion
Thus it was shown that a variety of agents from simple molecules to polymer macromolecules are used for surface modification. However, there are relatively few publications dealing with surface modification of silicon carbide powder.
Applied researches are carried outwith state financial support represented by the Ministry of Education of Russia under the Agreementon granting subsidies No.14.625.21.0003 of August 25,2014.(Unique identifier for Applied Scientific Researches(project) RFMEFI62514X0003).
References
- Wu, R.; Zhou, K.; Yue, C.Y.; Wei, J.; Pan, Y.Progress in Materials Science 2015, 72, 1-60
- Vadlapudi, V.;Kaladhar,D.S.V.G.K.;Behara, M.; Sujatha, B.; Naidu, G. K. Oriental Journal of Chemestry 2013, 29 (4), 1589-1595
- Ameen, S.;Shaheerakhtar, M.; Shin,H. S.Oriental Journal of Chemestry 2013, 29 (3), 837-860
- Morkoç, H.;Strite, S.; Gao, G. B.; Lin, M.E.;Sverdlov B.; Burns, M. J. Appl. Phys.1994, 76, 1363–1398.
- Goldberg, Y.,Levinshtein, M.E. and Rumyantsev, S.L.; Silicon carbide, John Wiley & Sons Inc., New York, (2001)
- Presser, R.; Nickel K.G. Crit. Rev. Solid State 2008, 33, 1–99.
- Fan, J.Y.; Wu, X.L.; Chu, P.K. Prog.Mater.Sci.2006, 51, 983–1031.
- Mélinon, P.;Masenelli, B.; Tournus, L.F.; Perez, A. Nature Mater.2007, 6, 479–490.
- Zhou W.M., Zhang, Y.F.,Niu X.M., Min G.Q.,One-dimensional nanostructures, Springer, (2008)
- Zekentes, K.;Rogdakis, K. J. Phys.D. Appl. Phys.2011, 44, 133001–133017.
- Maboudian, R.;Carraro, C.;Senesky, D.G.; Roper, C.S. J. Vac. Sci. Technol. A.2013, 31, 050805–050818.
- Nhuapeng.W.;Thamjaree, W;Kumfu, S.;Singjai, P.;Tunkasiri, T. Current Appl. Phys.2008, 8, 295−299.
- Meng, S.;Jin, G.G.; Wang, Y.Y.;Guo, X.Y. Mater.Sci. Eng.A2010, 527, 5761−5765.
- Dashb, S.;Swaina, S.K. Carbohyd.Polym.2013, 97, 758– 763.
- Wagner, G.; Schulz, D.;Siche, D. Progress in Crystal Growth and Characterization of Materials2003, 47, 139–165.
- Luthra K.L.Journal of the American Ceramic Society 1991, 74, 1095–1103.
- Iler R.А.,The chemistry of silica, John Wiley & Sons Inc., New York, (1979)
- Plueddemann, E.P.; Clark, H.A.; Nelson, L.E.; Hoffman K.R. Mod.Plast.1962, 39, 135
- Uyanik, M. PhD Thesis. Saarland University, Saarbrucken 2008, 199
- Kango, S. Progress in Polymer Science2013, 38, 1232-1261
- Rong, M.Z.; Zhang, M.Q.; Zheng; Y.X, Zeng; H.M.; Walter, R.; Friedrich, K. Polymer2001, 42, 167–183
- Tran, Y.;Auroy, P. Journal of the American Chemical Society2001, 123, 3644–3654.
- Mansky, P.; Liu, Y.; Huang, E; Russell, T.P.; Hawker, C. Science1997, 275, 1458–1460.
- Prucker, O;Ruhe, J. Macromolecules1998, 31, 592–601.
- Bazzar, M;Ghaemy, M.Compos SciTechnol.,2013, 86, 101−108.
- Gu, J.; Zhang, Q.; Dang, J.; Zhang, J.; Chen S. Polym. Bull.2009, 62, 689–697
- Omastova, M.;Boukerma, K.;Chehimi, M.M.;Trchova, M.Materials Research Bulletin2005, 40, 749–765
- Mavinakuli, P.; Wei, S.; Wang, Q.; Karki, A.B.;Dhage, S. Wang, Z.; Young, D.P.;Guo, Z.J. Phys. Chem. C 2010, 114, 3874–3882.
- JI,X.; Hao, H; Wu, Q.; Zhou B.; Sun, F. Journal of Wuhan University of Technology-Mater. Sci.Ed.2007, 22, 754-756
- Iijima, M.;Kamiya, H. J. Phys. Chem. C 2008, 112, 11786–11790.
- Rosso, M, Arafat, A.;Schroen, K.;Giesbers, M.; Roper, C. S.; Maboudian, R.; Zuilh of, H.Langmuir 2008, 24, 4007-4012.
- Novak,S.;Kovac,J.;Drazic,G.;Ferreira,J.M.F.;Quaresma, S.Journal of the European Ceramic Society 2007, 27, 3545–3550
- Prabhakaran,K.; James,J.;Pavithran, C.Journal of the European Ceramic Society 2003, 23, 379–385
- Zhang, Y.; Binner, J.J. Am. Ceram. Soc.2002, 85(3), 529–534
- Liden,E.;Bergstrem,L.;Persson,M.;Carlsson, R.Journal of the European Ceramic Society 1991,7, 361-368

This work is licensed under a Creative Commons Attribution 4.0 International License.









