An Efficient Synthesis of Functionalized 3-(α-amidobenzyl)-4-hydroxycoumarin Derivatives by ZnO Nanoparticles Promoted Condensation Reaction Between Aromatic Aldehyde, 4-hydroxycoumarin, and Amides
Hossein Anaraki-Ardakani1, Abbas charooseai2
1Department of Chemistry, College of Chemical Engineering, Mahshahr Branch, Islamic Azad University, Mahshahr, Iran
2Department of Chemistry, Omidiyeh Branch, Islamic Azad University, Omidiyeh, Iran.
Corresponding author E-mail hosseinanaraki@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/310324
Article Received on :
Article Accepted on :
Article Published : 24 Jul 2015
An efficient and green protocol for the synthesis of 3-(α-amidobenzyl)-4-hydroxycoumarin derivatives by one pot, three component coupling reaction of aromatic aldehyde, 4-hydroxycoumarin, and amides has been developed using ZnO nanoparticles (NPs) as the catalyst. The procedure is formed in high yields, short reaction time and an environmentally friendly specificity.
KEYWORDS:4-Hydroxycoumarin; ZnO nanoparticles (NPs); 3-(α-amidobenzyl)-4-hydroxycoumarins; aromatic aldehydes
Download this article as:| Copy the following to cite this article: Anaraki-Ardakani H, Charooseai A. An Efficient Synthesis of Functionalized 3-(α-amidobenzyl)-4-hydroxycoumarin Derivatives by ZnO Nanoparticles Promoted Condensation Reaction Between Aromatic Aldehyde, 4-hydroxycoumarin, and Amides. Orient J Chem 2015;31(3). |
| Copy the following to cite this URL: Anaraki-Ardakani H, Charooseai A. An Efficient Synthesis of Functionalized 3-(α-amidobenzyl)-4-hydroxycoumarin Derivatives by ZnO Nanoparticles Promoted Condensation Reaction Between Aromatic Aldehyde, 4-hydroxycoumarin, and Amides. Orient J Chem 2015;31(3). Available from: http://www.orientjchem.org/?p=9972 |
Introduction
Multi-component reactions have been attracting much interest from synthetic chemists because they provide simple one-pot routs for the synthesis of complex molecules from simple and easily available starting materials. These processes are single-step and don’t require separation and purification of intermediates and so save time, energy and raw materials 1. Recently, heterogeneous catalysts have attracted the attention of researchers due to their economic and industrial significance and published reports indicate that they scored over homogeneous catalysts. Among these, nanoscale heterogeneous catalysts are highly preferred as they offer high surface area and low-coordinated sites, which are responsible for the higher catalytic activity 2-4, having the advantage of easy product purification and reusability of the catalyst. The synthesis of coumarins and their derivatives has attracted considerable attention from organic and medicinal chemists for many years as a large number of natural products contain this heterocyclic nucleus. They are widely used as additives in food, perfumes, cosmetics, pharmaceuticals5 and optical brighteners6 and dispersed fluorescent and laser dyes 7. Among the various substituted coumarins, 3-(benzyl)-substituted 4-hydroxycoumarins represents a significant class of compounds as biologically active compounds,8, 9 (Fig. 1) and useful scaffolds, which can be used for the synthesis of 3,4-substituted compounds.10–13 The existing methods for the synthesis of 3-substituted 4-hydroxycoumarins include direct synthesis of the target compound14–17, or C3-alkylation/substitution of 4-hydroxycoumarin.18 Recently, we reported the reaction of 4-hydroxycoumarin, aromatic aldehydes, and acetonitrile in the presence of chlorosulfonic acid to produce 3-acetamido-alkyl-4-hydroxycoumarin derivatives. 19,20 also we reported the reaction of 4-hydroxycoumarin, aromatic aldehydes and amides in the presence p-toluene sulfonic acid in solvent-free conditions to produce 3-(α-amidobenzyl)-4-hydroxycoumarin derivatives 21 but these methodologies have been associated with some shortcomings such as long reaction times, and difficulty in recovery and reusability of the catalysts. One of the best ways to overcome these difficulties is to employ heterogeneous catalysis, as it enables a convenient recovery and reuse of the catalyst from the reaction mixture through simple filtration or decantation. 22–24.
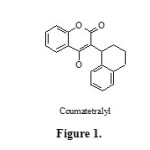 |
Figure 1 Click here to View figure |
Considering the above reports and in continuation of our research on multi-component reactions,25,26 Herein we have researched for three-component coupling of 4-hydroxycoumarin 1, aryl aldehydes 2, and amides 3, in the presence of ZnO nanoparticles as heterogeneous catalyst to the synthesis of 3-(α-amidobenzyl)-4-hydroxycoumarin derivatives 4 (Scheme 1).
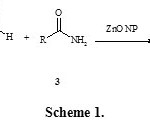 |
Scheme 1 Click here to View scheme |
Results and discussion
Firstly, in order to optimize the reaction conditions, the model reaction was carried out by using 4-hydroxycoumarin, 4-chlorobenzaldehyde, and acetamide under solvent-free conditions in the presence of different nanoparticle as catalysts and the results are listed in Table 1 .
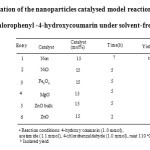 |
Table1: Optimization of the nanoparticles catalysed model reaction for synthesis of 3-acetamido-4-chlorophenyl -4-hydroxycoumarin under solvent-free condition a Click here to View table |
We examined this reaction in the presence of various nanoparticle catalysts in hand including Fe3O4 nanoparticles, MgO nanoparticles, NiO nanoparticles, ZnO nanoparticles (Table 1). It was showed that ZnO nanoparticle was the most efficient catalyst for the reaction in Solvent-Free condition (Table 1, entry 6). However, only a trace amount of the product was formed in the absence of catalyst (Table 1, entry 1).
Afterward, optimization of catalyst amounts was carried out in the model study by using different amounts of the ZnO NPs. The higher yield was obtained with increasing the amount of catalyst from 5 mol% to 15 mol%. However, further increase of the molar amount of the catalyst from 15 mol% to 25 mol% did not significantly increase the yield of the product (Figure 2). Hence, the optimum concentration of ZnO NPs was chosen 15 mol% in the model reaction.
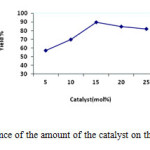 |
Figure2: Influence of the amount of the catalyst on the model reaction. Click here to View figure |
To improve the yield of the target product, we carried out the test reaction in presence of various solvents and the results are presented in Table 2. As can be seen from this table, solvent- free conditions accelerated the rate of reaction and also high yields were obtained for all products.
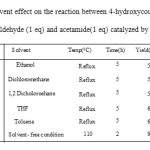 |
Table2: Solvent effect on the reaction between 4-hydroxycoumarin (1eq),4-chlorobenzaldehyde (1 eq) and acetamide(1 eq) catalyzed by ZnO(15mol%) Click here to View table |
To study the scope of the reaction, a series of aldehydes and amides were employed. The results are shown in Table 3. In all cases, aromatic aldehydes substituted with either electron-donating or electron-withdrawing groups underwent the reaction smoothly and gave the products in good yields. It could also be concluded that the aldehydes bearing electron-withdrawing groups required shorter time and gave higher yields (Table 3). Compounds 4a-j were known and their structures were deduced by comparison of melting points and spectral data with authentic samples. 19-21.
 |
Table3: Three-component reaction of aromatic aldehydes, 4-hydroxycoumarin and amides catalyzed by ZnO NP |
Although it is not clear how ZnOacts as a catalyst for the reaction, on the basis of the surface of metal oxides exhibit both Lewis acid and Lewis base character 27 and according to the literature survey,28-30 the suggested mechanism for the formation of the products is shown in Scheme 2. The reaction of 4-hydroxycoumarin with aromatic aldehydes in the presence of ZnO NPs catalyst is proposed to give 3-benzylidine-chroman-2,4-diones. These 3-benzylidine-chroman-2,4-diones, generated in situ, react with amide to form the 3-acetamido -alkyl-4-hydroxycoumarin products (Scheme 2 )
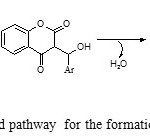 |
Scheme2: Suggested pathway for the formation of compounds 4a-j Click here to View scheme |
The reusability of the catalyst was tested in the synthesis of 3-(α-amidobenzyl)-4-hydroxycoumarin, as shown in Figure 2. The catalyst was recovered after each run, washed with ethanol, dried in an oven at 100 oC for 15 min prior to use and tested for its activity in the subsequent run. The catalyst was tested for 4 runs. It was seen that the catalyst displayed very good reusability (Figure 3).
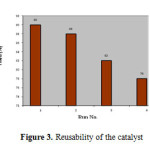 |
Figure3: Reusability of the catalyst Click here to View figure |
Experimental
Melting points were determined with an Electrothermal 9100 apparatus. Elemental analyses were performed using a Heraeus CHN-O-Rapid analyzer. Mass spectra were recorded on a FINNIGAN-MAT 8430 mass spectrometer operating at an ionization potential of 70 eV. IR spectra were recorded on a Shimadzu IR-470 spectrometer. 1H and 13C NMR spectra were recorded on Bruker DRX-500 Avance spectrometer at in DMSO-d6solution using TMS as internal standard. The chemicals used in this work purchased from fluka (Buchs, Switzerland) and were used without further purification.
Synthesis of nano-ZnO
Zinc oxide nanoparticles was prepared as previously described in the literature 31. In a typical procedure, zinc acetate (9.10 g, 0.05 mol) and oxalic acid (5.4 g, 0.06 mol) were combined by grinding in an agate mortar for 1 h at room temperature. Afterwards, the formed ZnC2O4_2H2O nanoparticles were calcinated at 450 oC for 30 min to produce ZnO nanoparticles under thermal decomposition conditions. The morphology, structure and size of the samples were investigated by Scanning Electron Microscopy (SEM). Fig. 4 indicates that the original morphology of the particle was approximately spherical with the diameter varying between 20 and 35 nm.
 |
Figure4: SEM image of synthesized ZnO nanoparticles. Click here to View figure |
General procedure
A mixture of 4-hydroxycoumarin (1.0 mmol), aromatic aldehyde (1.0 mmol), amide (1.0 mmol), and nano-ZnO (15 mol %) was heated at 110 ºC for 120-135 min. After completion of the reaction as indicated by TLC, the reaction mixture was cooled to room temperature. The solid residue was dissolved in hot ethanol and centrifuged to separate the catalyst. By recrystallization from ethanol, pure products were obtained.
N-[(4-Hydroxy-2-oxo-2H-chromen-3-yl)phenylmethyl propionamide (4j). white powder, m.p. 184 – 186oC, IR (KBr) (νmax cm-1): 3320 (NH), 1674, 1629 (2 C=O). Analyses: Calcd. for C19H17NO4: C, 70.58; H, 5.30; N, 4.33%. Found: C, 70.71; H, 5.22; N, 4.35%. MS (m/z, %): 323 (10). 1H NMR (500 MHz, d6-DMSO): δ = 0.91 (t,JHH = 7.6 HZ , 3 H, CH3), 2.27 (q, JHH = 7.6 HZ , 2 H, CH2), 6.58 (1 H, d, JHH = 8.8 Hz, CH), 7.20 (1 H, t, JHH = 7 Hz, CH of C6H5), 7.26-7.31 (4 H, m, 4 CH of C6H5), 7.36-7.41 (2 H, m, 2 CH of coumarin moiety), 7.65 (1 H, t,JHH = 8 Hz, CH of coumarin moiety), 8.05 (1 H, d,JHH = 8 Hz, CH of coumarin moiety), 8.38 (1 H, d,JHH = 8.8 Hz, NH), 10.15(1 H, broad, OH). 13C NMR (125.8 MHz, d6-DMSO): δ = 10.16 (CH3), 28.69 (CH2), 47.39 (CH), 106.69, 116.62, 116.77, 124.29, 124.50, 126.98, 152.82, 161.99, and 162.30 (carbons of coumarin moiety), 126.49, 128.17,132.87 and 141.07 (carbons of C6H5), 174.21 (NC=O).
Conclusion
In summary, we have described a simple, efficient, and environmentally benign one-pot procedure for the synthesis of 3-(α-amidobenzyl)-4-hydroxycoumarin derivatives by using catalytic amount of ZnO-NPs under solvent-free conditions. The advantages of the reported method are inexpensive and easily available starting materials, simple reaction conditions, low loading of catalyst, safety and reusability of catalyst, high yields, single-product reaction and simple workup procedure.
Acknowledgements
We gratefully acknowledge financial support from the Research Council of Islamic Azad University, Mahshahr branch.
References
- Domling, A.; Ugi, I. Angew. Chem. Int. Ed. Engl. 2000, 39, 3168-3210.
- Pacchioni, G. Surf. Rev. Lett. 2000, 7, 277-306.
- Knight, W. D.; Clemenger, K.; de Heer, W. A.; Saunders, W. A. M.; Chou Y.; Cohen, M. L. Phys. Rev. Lett. 1984, 52, 2141-2143.
- Kaldor, A.; Cox D.; Zakin, M. R. Adv. Chem. Phys. 1988, 70, 211-261.
- Gribble, G. W. In Comprehensive Heterocyclic Chemistry II, Vol. 2; A. R. Katriztky, C. W. Rees, , E. F. V. Scriven, Eds.; Elsevier: Oxford, 1996, 207.
- Zabradnik, M. The Production and Application of Fluorescent Brightening Agents; John Wiley & Sons: New York, 1992.
- Murray, R. D. H.; Mendez, J.; Brown, S. A. The natural coumarins: Occurrence, Chemistry and Biochemistry, John Wiley & Sons: New York, 1982.
- Raj, G.; Kumar, R.; McKinney. W. P. Am. J. Med. Sci. 1994, 307, 128-132.
- Hadler, M. R.; Shadbolt, R. S. Nature 1975, 253, 275-277.
- Estevez-Braun, A.; Gonzalez, A. G. Nat. Prod. Rep. 1997,14, 465-475.
- Clerici, O.; Porta,A. Synthesis 1993, 99-102.
- Mizuno, T.; Nishiguchi, I.; Hirashima, T.; Ogawa, A.; Kambe N.; Sonoda N. Synthesis 1988, 257-259.
- Wang, S.; Milne, G. W. A.; Yan, X.; Posey, I.; Nicklaus, M. C.; Graham, L.; Rice, W. G. J. Med. Chem. 1996, 39, 2047-2054.
- Dittmer, D. C.; Li, Q.; Avilov, D. V. J. Org. Chem. 2005, 70, 4682-4686.
- Kalinin, V.; Snieckus, V. Tetrahedron Lett. 1998, 39, 4999-5002.
- Kalinin, V.; Da Silva, A. J. M.; Lopes, C. C.; Lopes R. S. C.; Snieckus. V. Tetrahedron Lett. 1998, 39, 4995-4998.
- Davis, S. E.; Church, A. C.; Tummons R. C. and Beam, C. F. J. Heterocycl. Chem. 1997, 34 ,1159-1162.
- Reddy, R.; Srikanth, B.; Rao, N. N.; Shin, D. S. Tetrahedron 2008, 64 , 11666-11672.
- Anary-Abbasinejad, M.; Anaraki-Ardakani, H.; Saidipoor, A.; Shojaee, M. J. Chem. Res. 2007, 537-539.
- Anary-Abbasinejad, M.; Anaraki-Ardakani H.; Hassanabadi, A. Synth. Commun. 2008, 38, 3706-3710.
- Malekpour, M.; Anaraki-Ardakani H.; Noei. M. J. Chem. Res. 2012,11, 715-717.
- 22. Kantam, M. L.; Venkanna, G. T.; Sridhar, C.; Sreedhar B.; Choudary, B. M. J. Org. Chem. 2006, 71, 9522-9524.
- Islam, M.; Mondal, S.; Mondal, P.; Roy, A. S.; Tuhina, K.; Mobarok, M.; Paul, S.; Salam N.; Hossain, D. Catal. Lett. 2011, 141, 1171-1181.
- Bermudez, J. M.; Menéndez, J. A.; Romero, A. A.; Serrano, E.; Garcia-Martinez J.; Luque, R. Green Chem. 2013, 15, 2786-2792.
- Anaraki-Ardakani, H.; Noei M.; Tabarzad, A. Chinese Chemical Letters 2012, 23 , 45-48.
- Anaraki-Ardakani, H.; Noei, M. ; Karbalaei-Harofteh M. ; Zomorodbaksh, S. E-Journal of Chemistry 2012, 9, 2239-2244.
- Tanabe, K.; Solid Acids and Bases, Academic Press, New York, 1970.
- Azarifar, D., Yami, R. N. Heterocycles 2010, 81, 2063-2073.
- Shaterian , H. R.; Moradi, F. Research on Chemical Intermediates 2013, 1-3.
- Shaterian , H. R.; Azizi, K. Journal of Molecular Liquids 2013,183, 8-14.
- Shen, L.; Bao N.; Yanagisawa, K. Nanotechnology 2006, 17, 5117.

This work is licensed under a Creative Commons Attribution 4.0 International License.









