Adsorption of Si6O12 on CNTs in view point of NMR shielding tensors and NBO analysis: A novel material for drug delivery
Zahra Barmaki,*
Department of Chemistry, Science and Research Branch, Islamic Azad University, Tehran, Iran.
Correspondence Author Email: z.barmaki63@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/310353
Article Received on :
Article Accepted on :
Article Published : 10 Aug 2015
The interaction of the Si6O12 molecule over the CNTs have been investigated with density functional theory using HF and B3LYP method and 6-31G, 6-31G** and 6-311G** basis sets. We also analyze the electronic structure and charge Mulliken population for the energetically most favorable complexes. Our results indicate Si6O12 can form stable bindings with CNTs via the Oxygen. The NMR shielding tensors have been investigated. The same Study performed for Si7O14 and we found that this molecule can form stable bindings with CNTs via the Silicon site. Thus, we arrive at the prediction that the even number (Si6O6) has a different mechanism for adding to CNTs compare to add number such as Si7O14.
KEYWORDS:The interaction of the Si6O12; Adsorption; CNTs
Download this article as:| Copy the following to cite this article: Barmaki Z. Adsorption of Si6O12 on CNTs in view point of NMR shielding tensors and NBO analysis: A novel material for drug delivery. Orient J Chem 2015;31(3). |
| Copy the following to cite this URL: Barmaki Z. Adsorption of Si6O12 on CNTs in view point of NMR shielding tensors and NBO analysis: A novel material for drug delivery. Orient J Chem 2015;31(3). Available from: http://www.orientjchem.org/?p=10244 |
Introduction
SWCNTs have considered as the leading candidate for nano-device applications because of their one-dimensional electronic bond structure, molecular size, and biocompatibility, controllable property of conducting electrical current and reversible response to biological reagents hence SWCNTs make possible bonding to polymers and biological systems such as DNA and carbohydrates [28-35].
The carbon nanotube (CNT) is a representative nano-material. CNT is a cylindrically shaped carbon material with a nano-metric-level diameter [1-15].
Its structure, which is in the form of a hexagonal mesh, resembles a graphite sheet and it carries a carbon atom located on the vertex of each mesh. The sheet has rolled and its two edges have connected seamlessly [12-30].
Although it is a commonplace material using in pencil leads, its unique structure causes it to present characteristics that had not found with any other materials. CNT can be classified into single-wall CNT, double-wall CNT and multi-wall CNT according to the number of layers of the rolled graphite [22-42].
The type attracting most attention is the single-wall CNT, which has a diameter deserving the name of “nanotube” of 0.4 to 2 nanometers. The length is usually in the order of microns, but single-wall CNT with a length in the order of centimeters has recently released [42-50].
CNT can be classified into single-wall CNT, double-wall CNT and multi-wall CNT according to the number of layers of the rolled graphite. The type attracting most attention is the single-wall CNT, which has a diameter deserving the name of “nanotube” of 0.4 to 2 nanometers [50-63].
Its structure, which is in the form of a hexagonal mesh, resembles a graphite sheet and it carries a carbon atom located on the vertex of each mesh. The sheet has rolled and its two edges have connected seamlessly [60-74].
The length is usually in the order of microns, but single-wall CNT with a length about centimeters have recently released. The extremities of the CNT have usually closed with lids of the graphite sheet [71-81].
The lids consist of hexagonal crystalline structures (six-membered ring structures) and a total of six pentagonal structures (five-membered ring structures) placed here and there in the hexagonal structure [79-95]. The first report by Iijima was on the multiwall form, coaxial carbon cylinders with a few tens of nanometers in outer diameter. Two years later single walled nanotubes were reported.
The carbon nanotube (CNT) is a representative nano-material. CNT is a cylindrically shaped carbon material with a nano-metric-level diameter [94-98].
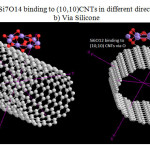 |
Figure 1: Si6O12 & Si7O14 binding to (10,10)CNTs in different direction a)via Oxygen b) Via Silicone |
In the NBO analysis, the interaction arising from electron delocalization are analyzed by selecting a number of natural bonding and anti-bonding orbitals that distort from the idealized Lewis structure, caused by interactions among them through hyper-conjugative or electrostatic interactions. In NBO analysis, the input atomic orbital basis set is transformed via natural atomic orbitals (NAOs) and natural hybrid orbital (NHOs) into NBO. In the present theoretical study, particular attention has been paid to the structural properties of The Si6O12-CNTs and Si7O14-CNTs systems. So, the NBO analysis gives supplementary information of the relative structural properties. It should be noted that conjugated systems, such as benzene and p-conjugated linear molecules, have been well studied with NBO analysis.
Computational Details
Calculations were performed using an all-electron linear combination of atomic orbitals Hatree–Fock (HF) and density functional theory (DFT) calculations using the Gaussian 03 package. The optimizations of antibiotics and Si6O12 are carried out including exchange and correlation contributions using Beckẻs three parameter hybrid and Lee-Yang-Parr (LYP) correlation [B3LYP]; including both local and non local terms. We have geometric optimization calculation at the HF/6-31G, HF/6-31G**, HF/6-311G**. We have also performed a geometric optimization calculation at the B3LYP/6-31G, B3LYP/6-31G** and B3LYP/6-31G** level.
The NMR isotropic shielding constants were calculated using the standard GIAO (Gauge-Independent Atomic Orbital) approach of Gaussian 03 program package.
a) The isotropic value σiso of the shielding tensor which can be defined as:
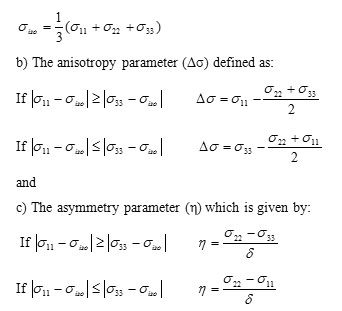
Results and Discussion
We studied about Si6O12 and Si7O14 molecules as the novel material for drug delivery.
Before and after connecting to the CNTs. NMR and NBO calculations were performed in electric field of charges. NMR and NBO parameters are listed in tables 1-5 in different levels and different basis sets.
HOMO and LUMO and Gap energy of MWC-Nano-cones are listed in Table1.
Contour line map of Localized orbital Locator (LOL) and Relief map (Shaded Surface map with projection) for electron density of Si6O12 are shown in Fig2 & 3. electron Localization Function of Si6O12 is shown in Fig 4.
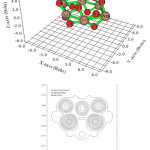 |
(Figure 2: Contour line map of Localized orbital Locator (LOL |
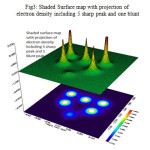 |
Figure 3: Shaded Surface map with projection of electron density including 5 sharp peak and one blunt Click here to View figure |
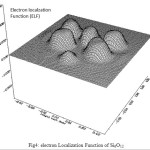 |
Figure 4: electron Localization Function of Si6O12 |
The situation of Si6O12 adsorption over CNTs and The situation of Si7O14 are shown in Fig1. Considering the optimized structure, the NMR shielding tensors were calculated then these parameters were used to show active sites in this structure. The results of бiso, бaniso, δ, Δб and η for this nano-cone in the same methods and basis sets are shown in table 2. Finally the charts of бiso, бaniso, δ and η for the atoms of Si6O12 Si7O14in 6-31G, 6-311G**, level of theory and B3LYP method. We can obtain the interesting results from the NMR charts. Comparison of these charts (бiso, бaniso, δ and η) shows that some of peaks in these charts are similar to each other. If these peaks are reviewed, we can understand which similar atoms are situated in the same peaks of different charts. The comparison of these peaks shows that three atoms are exactly repeated in бiso, бaniso, δ and η charts. These three atoms are the active sites in these structures. In general, the chart of electronic charge in different methods and basis sets is similar to the charts of NMR parameters Silicon atoms have more electrons than oxygen atoms.
So we can find that most chemical shielding. The concavity points are created due to the change of negative charge into a positive charge.
The electron density figure show that the mechanism of positive charge is different from negative charge. Positive and negative areas are completely different.
The results show that the heterocycle drugs connect to Si6O12 is stronger than Si7O14.
NBO analysis of the Si6O12 and Si7O14 system at the level of B3LYP/6-31g theory with different has been given in Table 3-6. The coefficients of s and p orbitals of both Si-O in Si6O12 and Si7O14 bonds can be distinguished based on these NBO data. Based on the constant values of the coefficients of linear combination of s and p orbitals of different bonds, a specific voltage differences could be expected. Summary of Natural Population analysis and Natural Population is listed in Table 3.Natural electron configuration of Valance and core electrons is listed in table 4. Occupancy, geminal, vicinal, energy, NBO, Principal delocalization is listed in Table 6.
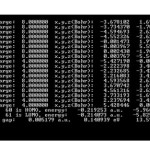 |
Table 1 Click here to View table |
Table 2: NMR parameters of Silicon andOxygen in Si6O12CNTs and Si7O14CNTs at the different levels
| η | δ | σaniso | σiso | Atom | B3LYP/6-311G** Si6O12CNTs |
|
0.25 |
-18.9 | 49.5 | 129.0 | (1)O | |
| 0.8 | -45. |
81.0 |
65.1 | (2)O | |
| 0.79 | -51.5 | 92.7 | 50.4 | (3)O | |
|
0.61 |
-133.2 | 277.6 | -26.7 | (4)O | |
| 0.3 | -22.8 | 57.2 | 58.3 | (5)O | |
| 0.64 | -59.0 | 120.3 | 177.0 | (6)O | |
| 0.64 | -159.8 | 324.0 | 76.9 | (7)O | |
| 0.22 | -20.0 | 53.3 | 108.6 | O(8) | |
| 0.87 | -13.0 | 21.9 | 159.1 | O(9) | |
| 0.88 | -54.8 | 92.1 | 47.2 | O(10) | |
| 0.72 | -18.8 | 35.9 | 171.8 | O(11) | |
| 0.08 | -55.1 | 45.0 | 203.8 | O(12) | |
| 0.35 | -16.2 | 16.5 | 179.4 | Si(1) | |
| 0.84 | -9.4 | 16.3 | 183.0 | Si(2) | |
| 0.48 | -24.2 | 26.9 | 159.3 | Si(3) | |
| 0.82 | -42.2 | 74.4 | 72.3 | Si(4) | |
| 0.28 | -19.3 | 49.8 | 164.6 | Si(5) | |
| 0.20 | -18.1 | 48.8 | 167.0 | Si(6) | |
| 0.83 | -19.3 | 33.7 | 175.5 | (1)O | B3LYP/6-31G Si6O12CNTs |
| 0.67 | -159.6 | 317.1 |
90.0 |
(2)O | |
| 0.76 | -43.4 | 80.5 | 79.1 | (3)O | |
|
0.68 |
-129.4 |
255.5 |
20.8 |
(4)O |
|
|
0.40 |
-19.2 |
46.03 |
94.2 |
(5)O |
|
|
0.50 |
-56.6 |
126.5 |
217.8 |
(6)O |
|
|
0.94 |
-56.0 |
81.9 |
75.7 |
(7)O |
|
|
0.12 |
-62.04 |
52.2 |
203.5 |
O(8) |
|
|
0.8 |
-9.4 |
16.9 |
187.5 |
O(9) |
|
|
0.8 |
-45.6 |
76.2 |
74.6 |
O(10) |
|
|
0.52 |
-72.7 |
83.3 |
131. |
O(11) |
|
|
0.76 |
-20.0 |
37.2 |
179.5 |
O(12) |
|
|
0.23 |
-15.1 |
14.1 |
200.3 |
Si(1) |
|
|
0.48 |
-58.0 |
131.7 |
207.4 |
Si(2) |
|
|
0.34 |
-20.3 |
20.4 |
185.8 |
Si(3) |
|
|
0.93 |
-42.5 |
67.8 |
77.8 |
Si(4) |
|
|
0.80 |
-65.5 |
88.5 |
160.7 |
Si(5) |
|
|
0.4 |
-24.0 |
56.1 |
142.0 |
Si(6) |
|
|
0.92 |
-30.9 |
44.7 |
90.5 |
(1)O |
B3LYP/6-311G** Si7O14CNTs |
|
0.64 |
152.4 |
308.8 |
97.0 |
(2)O |
|
|
0.75 |
-41.2 |
77.1 |
75.4 |
(3)O |
|
|
0.85 |
-33.2 |
46.3 |
93.9 |
(4)O |
|
|
0.42 |
-19.2 |
45.6 |
95.7 |
(5)O |
|
|
0.53 |
-53.4 |
117.5 |
220.2 |
(6)O |
|
|
0.95 |
-51.1 |
80.1 |
73.0 |
(7)O |
|
|
0.87 |
-34.3 |
48.4 |
83.3 |
O(8) |
|
|
0.77 |
-23.1 |
42.6 |
139.9 |
O(9) |
|
|
0.91 |
-57.1 |
92.7 |
49.9 |
O(10) |
|
|
0.19 |
-59.4 |
53.1 |
162.3 |
O(11) |
|
|
0.80 |
-45.1 |
81.0 |
65.1 |
O(12) |
|
|
0.51 |
101.5 |
-45.6 |
55.6 |
O(13) |
|
|
0.47 |
44.8 |
-19.6 |
155.3 |
O(14) |
|
|
0.30 |
-13.0 |
12.7 |
196.3 |
Si(1) |
|
|
0.70 |
-18.2 |
35.3 |
165.2 |
Si(2) |
|
|
0.44 |
-19.4 |
21.1 |
183.5 |
Si(3) |
|
|
0.89 |
-39.5 |
65.4 |
75.9 |
Si(4) |
|
|
0.71 |
-60.5 |
77.9 |
176.7 |
Si(5) |
|
|
0.26 |
-18.6 |
48.5 |
138.3 |
Si(6) |
|
|
0.13 |
-88.80 |
75.4 |
98.4296 |
Si(7) |
|
|
-0.44 |
-23.6 |
55.0 |
135.1 |
(1)O |
B3LYP/6-31G Si7O14CNTs |
|
-0.67 |
-128.7 |
256.6 |
32.5 |
(2)O |
|
|
-0.73 |
-43.2 |
81.7 |
67.6 |
(3)O |
|
|
-0.69 |
-137.3 |
269.41 |
8.15 |
(4)O |
|
|
-0.35 |
-20.6 |
51.01 |
86.57 |
(5)O |
|
|
-0.82 |
-22.5 |
39.8 |
156.8 |
(6)O |
|
|
-0.95 |
-54.1 |
85.0 |
65.2 |
(7)O |
|
|
0.06 |
-58.5 |
46.7 |
190.53 |
O(8) |
|
|
-0.80 |
-10.2 |
18.4 |
174.0 |
O(9) |
|
|
-0.82 |
-45.26 |
79.8 |
55.0 |
O(10) |
|
|
-0.26 |
-19.78 |
51.5 |
156.0 |
O(11) |
|
|
-0.83 |
-20.5798 |
35.8143 |
167.8563 |
O(12) |
|
|
0.45 |
-41.2 |
95.83 |
187.6 |
O(13) |
|
|
0.9 |
-70.7 |
115.6 |
29.1 |
O(14) |
|
|
0.39 |
-14.5 |
15.2 |
188.9 |
Si(1) |
|
|
-0.71 |
-18.6 |
35.9 |
156.6 |
Si(2) |
|
|
0.48 |
-21.2 |
23.6 |
175.7 |
Si(3) |
|
|
-0.89 |
-42.8 |
71.3 |
68.8 |
Si(4) |
|
|
0.73 |
-67.24 |
87.43 |
162.2 |
Si(5) |
|
|
-0.25 |
-18.9 |
49.5 |
129.0 |
Si(6) |
|
|
0.86 |
-41.3 |
70.4 |
41.287 |
Si(7) |
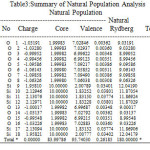 |
Table 3: Summary of Natural Population Analysis Natural Population Click here to View table |
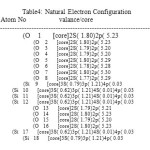 |
Table 4: Natural Electron Configuration A to m No valance/core |
 |
Table 5: Natural Bond Orbital Analysis Occupancies Lewis Structure Low High Click here to View table |
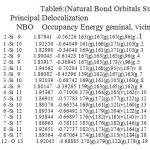 |
Table 6: Natural Bond Orbitals Summary) Principal Delocalization NBO Occupancy Energy geminal, vicinal, remote. Click here to View table |
References
- Hartogh, Paul; Lis, Dariusz C.; Bockelée-Morvan, Dominique; De Val-Borro, Miguel; Biver, Nicolas; Küppers, Michael; Emprechtinger, Martin; Bergin, Edwin A. et al. Nature. 2011, 478 (7368), 218–220.
- Hersant, Franck; Gautier, Daniel; Hure, Jean‐Marc. The Astrophysical Journal, 2001, 554 (1), 391–407.
- Altwegg, K.; Balsiger, H.; Bar-Nun, A.; Berthelier, J. J. et al.. Science, 2014, 347: 1261952..
- Rubio Angel; Corkill , Jennifer L.; Cohen , Marvin L. Phys. Rev. 1994. B, 49, 5081
- Bourgeois,L.; Bando,Y.; Han, W.Q.; Sato, T.Phys. Rev. 2000, B, 61, 7686.
- Terauchi, M.; Tanaka, K.; Suzuki, A.; Ogino, K.; Kimura, Chem. Phys. Lett. 2000, 324, 359.
- Sachdeva, H, Frank Müllera, F .; Stefan Hüfnerb, S. Diamond and Related Materials, 2010. 19, 1027-1033.
- Massimo, Fusaro .:Quantum Matter,2014, 3, 481-487
- Micheal Arockiaraj , Rev. Theor. Sci. 2014, 2, 261-273
- Kroto, H. W.; Health, J. R.; O’Brien, S. C.; Curl, R. F.; Smalley, R. E. C60: Buckminsterfullerene. Nature (London), 1985, 318, 162
- Iijima Sumio. Nature (London), 1991. 354, 56.
- Chopra, Nasreen G.; Luyken , R. J.; Cherrey , K.; Crespi , Vincent H.; Cohen, Marvin L.; Louie, Steven G.; Zettl , A. Science,1995, 269, 966.
- Monajjemi, M.; Baei, M.T.; Mollaamin, F. Russian Journal of Inorganic Chemistry. 2008, 53 (9), 1430-1437
- Monajjemi, M.; Rajaeian, E.; Mollaamin, F.; Naderi, F.; Saki, S. Physics and Chemistry of Liquids. 2008, 46 (3), 299-306
- Monajjemi, M.; Seyed Hosseini, M. Journal of Computational and Theoretical Nanoscience .2013 ,10 (10), 2473-2477
- Yahyaei ,H.; Monajjemi, M. Fullerenes, Nanotubes, and Carbon Nanostructures. 2014, 22(4), 346–361
- Monajjemi, M .; Jafari Azan, M.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures. 2013, 21(6), 503–515
- Bhupesh, Bishnoi .; Bahniman ,Ghosh .Quantum Matter.2014, 3, 469-475
- Sule, Celasun , Rev. Theor. Sci.2013, 1, 319-343
- Akshay kumar, Salimath .; Bahniman, Ghosh, Quantum Matter, 2014, 3, 72-77
- Nafisi, S.; Monajemi, M.; Ebrahimi, S. Journal of Molecular Structure. 2004,705 (1-3) 35-39
- Monajjemi , M.; Baheri ,H.; Mollaamin ,F. Journal of Structural Chemistry.2011 52(1), 54-59
- Monajjemi, M.; Seyed Hosseini, M.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures. 2013, 21, 381–393
- Monajjemi, M.; Boggs, J.E. J. Phys. Chem. A, 2013, 117, 1670 −1684
- Davide Fiscaletti and Amrit Sorli ,Quantum Matter,2014 3, 200-214
- Monajjemi , M.; Honaparvar , B.; Khalili Hadad ,B.; Ilkhani ,AR.; Mollaamin, F. Afr. J. Pharm. Pharmacol .2010, 4 (8), 521-529
- Bjürn Piglosiewicz, Jan Vogelsang.;Slawa Schmidt.; Doo Jae Park.; Petra Groß, .; Christoph Lienau ,Quantum Matter,2014 ,3, 297-306
- Monajjemi, M. Chemical Physics. 2013, 425, 29-45
- Fazaeli ,R.; Monajjemi ,M.; Ataherian ,F.; Zare, K. Journal of Molecular Structure: THEOCHEM.2002, 581 (1), 51-58
- Monajjemi, M.; Mollaamin, F, J Clust Sci, 22(2011)673.
- Medhat Ibrahim and Hanan Elhaes ,Rev. Theor. Sci. 2013, 1, 368-376
- Anurag Srivastava.; Nileshi Saraf.; A. K. Nagawat , Quantum Matter,2013, 2, 401-407
- IIJIMA Sumio.; YUDASAKA Masako.; NIHEY Fumiyuki.; NEC TECHNICAL JOURNAL,2007, 2,1,
- S. Iijima and T. Ichihasi, Nature,1993,363, 603
- D. S. Bethune.;C. H. Kiang.; M. S. deVries.; G. Gorman, R. Savoy.; J. Vazques.; R. Beyers, Nature ,1993, 363, 605
- Monajjemi, M .; Sobhanmanesh, A .; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures,2013, 21 47–63
- Monajjemi ,M.; Karachi ,N.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures, ,2014, 22: 643–662
- Monajjemi, M.; Mahdavian, L.; Mollaamin, F.: Bull .Chem.Soc.Ethiop ,2008, 22(2),1-10.
- V. Tozzini.; F. Buda.; A. Fasolino.; physical review letters ,2000,21,85
- G. Seifert.; P. W. Fowler.; Mitchell, D.; Porezag, D.; Frauenheim, Th. Chem. Phys. Lett. 1997, 268, 252
- Mollaamin ,F.; Baei, MT.; Monajjemi, M.; Zhiani , R.; Honarparvar , B.; Russian Journal of Physical Chemistry A, Focus on Chemistry,2008, 82 (13), 2354-2361
- Monajjemi, M.; Ghiasi, R. ;Ketabi, S. Journal of Chemical Research.2004, 1: 11-18
- Mahdavian,L.; Monajjemi, M.; Mangkorntong ,N.Fullerenes, Nanotubes and Carbon Nanostructures,2009, 17 (5), 484-495
- Mollaamin, F.; Gharibe, S.; Monajjemi, M. Int. J. Phy. Sci , 2011,6, 1496-1500
- Monajjemi, M .; Faham, R.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures , 2012 20, 163–169
- Monajjemi, M.; Khaleghian, M.; Tadayonpour, N.; Mollaamin, F. International Journal of Nanoscience, 2010, 9 (05), 517-529
- Mollaamin , F .; Najafi ,F.; Khaleghian, M.; Khalili Hadad, B.; Monajjemi ,M. Fullerenes, Nanotubes, and Carbon Nanostructures,2011 19, 653–667
- Monajjemi, M.; Chegini , H. ; Mollaamin , F. ; Farahani ,P, Fullerenes, Nanotubes, and Carbon Nanostructures.2011,19, 469–482
- Monajjemi, M.; Yamola ,H.; Mollaamin,F. Fullerenes, Nanotubes, and Carbon Nanostructures, 2014, 22, 595–603
- Monajjemi, M.; Heshmata, M.; Haeri, HH , Biochemistry (Moscow),2006, 71 (1), S113-S122
- Monajjemi, M. Theor Chem Acc , 2015, 134:77 DOI 10.1007/s00214-015-1668-9
- A. Rubio.; J.L. Corkill.; M.L. Cohen.; Phys. Rev. B ,1994, 49, 5081
- X. Blasé.; A. Rubio.; S.G. Louie.; M.L. Cohen.; Europhys. Lett, 1994.28, 335.
- N.G. Chopra.; J. Luyken.; K. Cherry.; V.H. Crespi.; M.L. Cohen,S.G. Louie.; A. Zettl, Science.1995 , 269, 966
- N.G. Chopra.;A. Zettl.;Solid State Commun.1998 ,105, 297
- J. Cumings.; A. Zettl, Solid State Commun.2004, 129, 661
- R. Ma.; Y. Bando.; H. Zhu.; T. Sato, C. Xu, D. Wu, J. Am.Chem. Soc.2002, 124, 7672,
- P.W. Fowler, K.M. Rogers, G. Seifert, M. Terrones, and H. Terrones, Chem. Phys. Lett. 1999, 299, 359
- Monajjemi, M .; Falahati, M.; Mollaamin, F.; Ionics, 2013 , 19, 155–164
- Monajjemi , M.; Heshmat ,M.; Aghaei , H.; Ahmadi , R.; Zare,K. Bulletin of the Chemical Society of Ethiopia, 2007, 21 (1)
- Monajjemi , M.; Lee, V.S. ; Khaleghian, M.; B. Honarparvar, B.; F. Mollaamin, F, J. Phys.Chem. C. 2010, 114 (2010) 15315
- A. Rubio, J.L. Corkill, M.L. Cohen, Phys. Rev. B.1994, 49,5081
- X. Blase, A. Rubio, S.G. Louie, M.L. Cohen, Europhys. Lett. 1994, 28 335.
- N.G. Chopra, J. Luyken, K. Cherry, V.H. Crespi, M.L. Cohen,S.G. Louie, A. Zettl, Science ,1995, 269, 966.
- O.R. Lourie, C.R. Jones, B.M. Bartlett, P.C. Gibbons, R.S. Ruoff,W.E. Buhro, Chem. Mater.2000, 12 ,1808;
- R. Ma, Y. Bando, T.Sato, Chem. Phys. Lett.2001, 337 ,61.
- W. Han, Y. Bando.; K. Kurashima.; T. Sato, Appl. Phys. Lett.1998, 73, 3085;
- (a) D. Golberg.; Y. Bando.; M. Eremets.; K. Takemura.; K. Kurashima.;H.Yusa, Appl. Phys. Lett.1996 ,69, 2045
- D.P. Yu, X.S. Sun, C.S. Lee, I. Bello, S.T. Lee, H.D. Gu, K.M. Leung, G.W. Zhou, Z.F. Dong.; Z. Zhang.; Appl. Phys. Lett.1998, 72 , 1966.
- F.Jensen.; H.Toftlund, Chem. Phys. Lett.1993, 201,89 , 94
- Monajjemi, M .; Aghaie , H.; Naderi , F. Biochemistry (Moscow).2007, 72 (6), 652-657
- Monajjemi, M. Journal of Molecular Modeling , 2014, 20, 2507
- Davide Fiscaletti,Rev. Theor. Sci.2013 1, 103-144
- Monajjemi , M.; Chahkandi ,B.; Zare,K.; Amiri, A. Biochemistry (Moscow),2005 70 (3), 366-376
- Monajjemi, M .; Afsharnezhad ,S.; Jaafari , M.R.; Abdolahi ,T.; Nikosade ,A.; Monajemi ,H.; Russian Journal of physical chemistry A, 2007, 2,1956-1963
- Monajjemi, M.; Khaleghian, M, Journal of Cluster Science. 2011, 22 ( 4 ), 673-692
- Mollaamin , F.; Monajjemi , M , Journal of Computational and Theoretical Nanoscience. 2012, 9 (4) 597-601
- Monajjemi, M. Struct. Chem, 2012, 23 551.
- Jon M. Matxain.; Jesus M. Ugalde.; M. D. Towler.; and R. J. Needs.; J. Phys. Chem. A 2003, 107, 10004-10010
- WU Haishun.;XU Xiaohong,; JIAO Haijun, Zhang Fuqiang .; JIA Jianfeng. Chinese Science Bulletin. 2003, 48, 11 1102 1107
- Monajjemi, M .; Ketabi ,S.; Amiri, A. Russian Journal of Physical Chemistry , 2006, 80 (1), S55-S62
- M. Monajjemi .; Robert Wayne Jr, J.E. Boggs, Chemical. Physics. 433 (2014) 1-11
- Jon M. Matxain.; Jesus M. Ugalde.; M. D. Towler.; and R. J. Needs.; J. Phys. Chem. A 2003, 107, 10004-10010
- WU Haishun.; XU iaohong.; JIAO Haijun, ZHANG Fuqiang .; JIA Jianfeng Chinese Science Bulletin 2003, 48 (11), 1102 1107
- Monajjemi , M.; Honarparvar, B.; Monajemi, H.;. Journal of the Mexican Chemical Society, 2006, 50 (4), 143-148
- Monajjemi ,M.; Mollaamin ,F. Journal of Computational and Theoretical Nanoscience,2012 ,9 (12) 2208-2214
- Monajjemi, M.; Mahdavian, L.; Mollaamin, F.; Honarparvar, B. Fullerenes, Nanotubes and Carbon Nanostructures, 2010, 18, 45–55
- Friedrich, B.; J.D. Weinstein.; R. Decarvalho .; J.M. Doyle.;. Trap. J. Chem. Phys. 1999, 110,2376-2383.
- Frischend, M.J.; J.B. Foresman, 1995. Gaussian 94 user’ reference (Gaussian, Inc., Pittsburgh).
- Ghalandari, B.; Monajjemi, M.; Mollaamin, F.; Journal of Computational and Theoretical Nanoscience, 2011 8, 1212–1219
- Monajjemi , M.; Khosravi , M.; Honarparvar, B.; Mollaamin, F.; International Journal of Quantum Chemistry, 2011, 111, 2771–2777
- Monajjemi, M.; Rajaeian, E.; Mollaamin, F. Physics and Chemistry of Liquids,2008, 46 299.
- Tahan, A .; Monajjemi, M. Acta Biotheor, 2011, 59, 291–312
- Monajjemi, M.; Farahani, N.; Mollaamin, F. Physics and Chemistry of Liquids, 2012, 50(2) 161–172
- Monajjemi, M.; Razavian, M.H.; Mollaamin,F.; Naderi,F.; Honarparvar,B.; Russian Journal of Physical Chemistry A , 2008 , 82 (13), 2277-2285
- Mollaamin , F.; Varmaghani , Z.; Monajjemi , M, Physics and Chemistry of Liquids. 2011, 49 318
- Monajjemi, M.; Honarparvar, B.; H. Haeri, H.; Heshmat, M.; Russian Journal of Physical Chemistry C, 2008, 80(1),S40-S44.
- Naghsh,F, oriental journal of chemistry, 2015, 31(1) 465-478
- Chitsazan, A, oriental journal of chemistry, 2015, 31(1) 393- 408

This work is licensed under a Creative Commons Attribution 4.0 International License.









