Adsorption of Congo Red by Ni/Al-CO3: Equilibrium, Thermodynamic and Kinetic Studies
N. Ayawei1, A. T. Ekubo2, D. Wankasi,1,3. and E. D. Dikio1,3.
1Department of Chemical Sciences, Niger Delta University, Wilberforce Island, Bayelsa State, Nigeria 2Department of Chemical Sciences, Federal University of Technology, Otuoke, Bayelsa State, Nigeria 3Applied Chemistry and Nanoscience Laboratory, Department of Chemistry, Vaal University of Technology, P. O. Box X021, Vanderbijlpark, South Africa. Corresponding author: ayawei4acad@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/310307
Article Received on :
Article Accepted on :
Article Published : 24 Jul 2015
Experimental investigations were carried out using Ni/Al-CO3 layered double hydroxide as adsorbent for removal of toxic anionic dye namely Congo red from aqueous solutions. The effect of contact time, initial dye concentration and temperature were experimentally studied in batch mode to evaluate the kinetic, equilibrium and thermodynamic parameters of the adsorption process. Experimental results revealed that the degradation of the dye is mostly dependent on temperature. The dye degradation process obeyed the zero-order kinetic model, first-order kinetic model, second-order kinetic model, pseudo second order kinetic and third order kinetic model with correlation coefficient values 1, 0.9998, 0.9999, 0.9999 and 0.9997 respectively. Langmuir, Freundlich, Temkin and Dubinin-Kaganer-Radushkevic isotherms were applied to the equilibrium data and was well described by all. Thermodynamic studies showed congo red adsorption on the layered double hydroxide was endothermic and spontaneous in nature. The results indicate that layered double hydroxide could be employed as alternative for removal of anionic dyes from industrial wastewater.
KEYWORDS:Congo Red; Layered double hydroxides; Kinetic; Dye; Adsorption; isotherms; thermodynamics
Download this article as:| Copy the following to cite this article: Ayawei N, Ekubo A. T, Wankasi D, Dikio E. D. Adsorption of Congo Red by Ni/Al-CO3: Equilibrium, Thermodynamic and Kinetic Studies. Orient J Chem 2015;31(3). |
| Copy the following to cite this URL: Ayawei N, Ekubo A. T, Wankasi D, Dikio E. D. Adsorption of Congo Red by Ni/Al-CO3: Equilibrium, Thermodynamic and Kinetic Studies. Orient J Chem 2015;31(3).. Orient J Chem 2015;31(3). Available from: http://www.orientjchem.org/?p=9995 |
Introduction
It was estimated that 10-20 % of dye was lost during the dyeing process and released as effluent [1]. Due to their chemical structures, dyes are resistant to fading on exposure to light, water and many chemicals and, therefore, are difficult to be decolorized once released into the aquatic environment [2]. Dyes can also cause allergic dermatitis and skin irritation. Some of them have been reported to be carcinogenic and mutagenic for aquatic organisms [3]. Therefore, it is very important to develop new systems that can be used for removing dyes from waters.
The treatment of dyes in industrial wastewater possesses several problems since dyes are generally difficult to biodegradate and photodegradate. Many different techniques including cloud point extraction, oxidation processes, nanofiltration, ozonation and coagulation have been used for the removal of colored dyes from wastewater [4-7]. However, adsorption is the most popular physicochemical treatment for the removal of dissolved organics from waters.
Layered double hydroxides (LDH) or anionic clays are lamellar ionic compounds, containing a positively charged layer and exchangeable anions in the interlayer [8]. They consist of brucite-like layers, with a partial MII for MIII substitution, leading to an excess of positive charge compensated with anions situated in the interlayer together with water molecules. These materials can be represented by the general formula
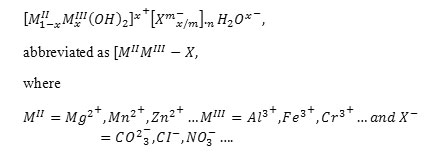
The flexibility of the structure of these materials as well as their high anionic exchange capacity make them suitable for many applications [9,10], such as the sorption of many inorganic [11,12,13,14] and organic [15,16,17,18,19,20] anions, potential contaminants of waters. Some authors have reported about LDH containing chelating agents such as etilendiaminetetraacetate, edta [21] and nitrilotriacetate, NTA, [22] as well as the metal cations uptake by these materials [23,24,25].
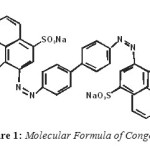 |
Figure1: Molecular Formula of Congo Red Click here to View figure |
Materials and Methods
Synthesis of Ni/Al -CO3
Carbonate form of Ni/Al LDH was synthesized by co-precipitation method. A 50 ml aqueous solution containing 0.3 M Ni (NO3)2.6H2O and 0.1 M Al (NO3)3.9H2O with Ni/Al ratios 2:1, was added drop wise into a 50 ml mixed solution of (NaOH (2M) + Na2CO3 (1M) with vigorous stirring and maintaining a pH of greater than 10 at room temperature. After complete addition which last between 2 hours 30 minutes to 3 hours, the slurry formed was aged at 60°C for 18 hours. The products were centrifuged at 5000 rpm for 5 minutes, with distilled water 3-4 times and dried by freeze drying.
Characterization of Layered Double Hydroxide
X-ray diffraction (XRD) pattern of the sample was characterized by using a Shimadzu XRD-6000 diffractometer, with Ni-filtered Cu-Kα radiation (λ= 1.54 A°) at 40 kV and 200 mA. Solid samples were mounted on alumina sample holder and basal spacing (d-spacing) was determined via powder technique. Samples scan were carried out at 10-60°, 2θ / min at 0.003° steps.
FTIR spectrum was obtained using a Perkin Elmer 1725X spectrometer where samples will be were finely ground and mixed with KBr and pressed into a disc. Spectrums of samples were scanned at 2 cm-1 resolution between 400 and 4000cm-1.
FESEM/EDX was obtained using Carl Zeiss SMT supra 40 VPFESEM Germany and inca penta FET x 3 EDX, Oxford. It was operated at extra high tension (HT) at 5.0 kV and magnification at 20000X. FESEM uses electron to produce images (morphology) of samples and was attached with EDX for qualitative elemental analysis.
Preparation of Congo Red Solution
Congo red (CI=22120) was supplied by Merck (Mumbai, India). A stock solution of CR dye was prepare (100mg/L) by dissolving a required amount of dye powder in deionized water. The stock solution was diluted with deionized water to obtain the desire concentration ranging from 20 to 40mg/L.
The concentration of CR in the experimental solution was determined from the calibration curve prepared by measuring the absorbance of different known concentrations of CR solutions at λmax =497 nm using a UV-vis spectrophotometer (Shimadzu, Kyoto, Japan). The pH meter using a combined glass electrode (model HI 9025C, Hanna instruments, Singapore).
Experimental Procedure
Batch adsorption experiments were carried out to study the effect of initial congo red concentration, contact time and temperature on the adsorption of Congo red on the layered double hydroxide. Adsorption studies were carried out using 25 ml of each dye solution and 0.2g of the adsorbent. At the end of each experiment, the content of each tube was filtered using a Whatman No 14 filter paper after which the concentration of residual congo red was determined by UV-Vis spectrophotometer analysis. All experiments were carefully conducted to acquire good result.
In order to determine the rate of adsorption, experiments were conducted with different initial concentrations of dyes ranging from 20 to 40 mg/L. All other factors are kept constant.
The effect of period of contact on the removal of the dye on adsorbent in a single cycle was determined by time intervals of 10, 20 and 30 minutes.
The adsorption experiments were performed at three different temperatures viz., 40, 60 and 80˚C in a thermostat attached with a shaker (Remi make). The constancy of the temperature was maintained with an accuracy of ± 0.5º C.
The equilibrium adsorption capacity and the yield of adsorption were calculated respectively by equations 1 and 2 below:

where Cinit and Ceql are, respectively, the initial and equilibrium concentrations of metal ions in solution (mmol/l) and m is the layered double hydroxide dosage (g/l).
Isotherms Analysis
To determine the equilibrium parameters of the adsorption process four isotherms namely, Freundlich, Langmuir, Temkin and Dubinin–Kaganer–Radushkevich (DKR) were applied to test the experimental data.
For the Freundlich isotherm the In-In version was used:
![]()
The Langmuir model linearization (a plot of 1/qeql vs 1/Ceql) was expected to give a straight line with intercept of 1/qmax:
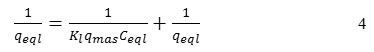
The essential characteristics of the Langmuir isotherm were expressed in terms of a
dimensionless separation factor or equilibrium parameter Sf.

With Co as initial concentration of Congo Red in solution, the magnitude of the parameter Sf provides a measure of the type of adsorption isotherm. If Sf> 1.0, the isotherm is unfavourable; Sf = 1.0 (linear); 0< Sf < 1.0(favourable) and Sf=0 (irreversible).
The DKR isotherm is reported to be more general than the Langmuir and Freundlich isotherms. It helps to determine the apparent energy of adsorption. The characteristic porosity of adsorbent toward the adsorbate and does not assume a homogenous surface or constant sorption potential [26].
The Dubinin–Kaganer–Radushkevich (DKR) model has the linear form
Inqe = InXm – βε2 6
where Xm is the maximum sorption capacity, β is the activity coefficient related to mean sorption energy, and ε is the Polanyi potential, which is equal to
ε = RTIn(1+1/Ce) 7
where R is the gas constant (kJ/kmol) . The slope of the plot of ln e q versus ε 2 gives β (mol2/J2 ) and the intercept yields the sorption capacity, Xm (mg/g). The values of β and Xm, as a function of temperature are listed in table 1 with their corresponding value of the correlation coefficient, R2. It can be observed that the values of β increase as temperature increases while the values of Xm decrease with increasing temperature.
The values of the adsorption energy, E, was obtained from the relationship [27]
E = (2β)-1/2 8
The Temkins isotherm model was also applied to the experimental data, unlike the Langmuir and Freundlich isotherm models, this isotherm takes into account the interactions between adsorbents and metal ions to be adsorbed
and is based on the adsorption that the free energy of adsorption is simply a function of surface coverage [28]. The linear form of the Temkins isotherm model equation is given in (9).
qe = BlnA + BlnCe 9
Where B= [RT/bT] in (J/mol) corresponding to the heat of adsorption, R is the ideal gas constant, T(K) is the absolute temperature, bT is the Temkins isotherm constant and A (L/g) is the equilibrium binding constant corresponding to the maximum binding energy.
Kinetic Parameters
The experimental data were further subjected to certain kinetic parameters.
Zero-order kinetic model,
qt = q0 + K0t 10
First-Order Kinetic model,
Inqt = Inq0 +Ktt 11
Second-Order Kinetic model,
![]()
Third-order kinetic model
![]()
Pseudo-second order model
where qo (mg/g) and qt (mg/g) are the adsorbed amounts of CR at equilibrium and time t (min); Ko, K1, K2 and K3 are the adsorption rate constants for the kinetic models.
Thermodynamic parameters
Thermodynamic parameters such as change in Gibb’s free energy DGo, enthalpy DHo and entropy DSo were determined using the following equation [9]:
![]()
where Kd is the apparent equilibrium constant, qeql (or [Congo Red]uptake); is the amount of metal adsorbed on the unitary sorbent mass (mmol/g) at equilibrium and Ceql (or [Congo Red]eql) equilibrium concentrations of metal ions in solution (mmol/l), when amount adsorbed is equals qeql; -qeql/Ceql relationship depends on the type of the adsorption that occurs, i.e. multi-layer, chemical, physical adsorption, etc.
The thermodynamic equilibrium constants (Kd) of the Congo Red adsorption on studied layered double hydroxide were calculated from the intercept of the plots of ln (qeql/Ceql) vs. qeql.
Then, the standard free energy change DGo, enthalpy change DH0 and entropy change DSo were calculated from the Van’t-Hoff equation [9].
ΔG0 = – RT In Kd 16
where Kd is the apparent equilibrium constant; T is the temperature in Kelvin and R is the gas constant (8.314 Jmol-1K-1):
The slope and intercept of the Van’t-Hoff plot [8] of ln Kd vs. 1/T were used to determine the values of ΔHo and ΔSo,
![]()
Then, the influence of the temperature on the system entropy was evaluated using the equations [11]. The plot of ΔGo vs. t also give the result of ΔHo and ΔHoSo.
ΔGo ΔHo – TΔSo 18
The thermodynamic parameters of the adsorption were also calculated by using the Langmuir constant (KL), Freundlich constants (KF) for the equations [12–14] instead of (Kd). The obtained data on thermodynamic parameters were compared, when it was possible.
The differential isosteric heat of adsorption (DHx) at constant surface coverage was calculated using the Clausius-Clapeyron equation [12]:
![]()
Integration gives the following equation [10]:
![]()
where K is a constant. The differential isosteric heat of adsorption was calculated from the slope of the plot of ln(Ceql) vs 1/T and was used for an indication of the adsorbent surface heterogeneity. For this purpose, the equilibrium concentration (Ceql) at constant amount of adsorbate adsorbed was obtained from the adsorption isotherm data at different temperatures.
Results and Discussion
Characterization of LDH
FT-IR
Figure 2 shows the pre and post adsorption spectra of congo red on Ni/Al-LHD. The strong bond around 3400cm-1 as shown in 4(a) is associated with the stretching vibration of OH groups in the brucite like layer and interlayer inter molecules.
The broadening of the bond was attributed to the hydrogen-bond formation. Less intense absorption bond around 1650 – 1500 cm-1 was assigned to the bending vibration of the interlayer water molecules. The carbonate ion peak is around 1400cm-1 which is consistent with layered double hydroxides. Figure 4(b) shows sharp peak around 733cm-1 which is characteristic of bending vibration of primary amine and phosphate bond stressing between 1100cm-1 – 1200cm-1. This shows adsorption actually occurs via formation of complex between the congo red and the layered double hydroxides [29].
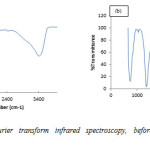 |
Figure2: Ni/Al-C03 Fourier transform infrared spectroscopy, before (a) and after (b) adsorption studies. Click here to View figure |
XRD
Figure 3 shows the XRD patterns of the Ni/Al. The basal reflections are observed at low 2q values and weaker non-basal reflections at higher 2q. The reflections in the Ni-Al based layered double hydroxide is indexed to rhombohedral symmetry (space group R-3m). Three successive reflections at 8.5Å, 22.34Å and 34.98Å respectively, which could be indexed to (003) and (006) and (009) planes corresponding to d-spacing of 1.039nm, 0.3975nm and 0.2562nm respectively.
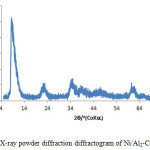 |
Figure3: X-ray powder diffraction diffractogram of Ni/Al2-CO3 Click here to View figure |
SEM
Figures 4 clearly shows the pre & post adsorption SEM images. The SEM image of post adsorption shows coverage of available pores in relation to pre-adsorption image.
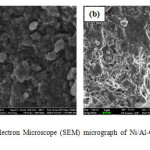 |
Figure4: Scanning Electron Microscope (SEM) micrograph of Ni/Al-CO3 before (a) and after (b) adsorption studies. Click here to View figure |
Effect of Concentration
Removal efficiency of Congo Red by adsorbents is illustrated in figure 5. It shows that removal efficiency decreased with increasing of initial concentration (52.5%, 50% and 47.8%) respectively, this is probably due to rapid adsorption at all available site and relatively small amount of adsorbent that was used, an increase in the amount of adsorbent may therefore reverse adsorption trend.
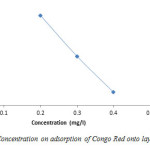 |
Figure5: Effect of Concentration on adsorption of Congo Red onto layered double hydroxide Click here to View figure |
Isotherm Analysis
To investigate an interaction of adsorbate molecules and adsorbent surface, four well-known models, the Langmuir, Freundlich, Dubinin-kaganer-radushkevic and Temkin isotherms, were selected to explicate LDH interaction in this study.
The Langmuir plot in figure 6 fitted the experimental data with R2= 0.9924 and therefore, confirm monolayer coverage.
The influence of isotherm shape on whether adsorption is favourable or unfavourable has been considered. For a Langmuir type adsorption process, the isotherm shape can be classified by a dimension less constant separation factor (RL), given by Eq. (4). The calculated value of RL from figure 5 is 0.7, which is within the range of 0–1, thus confirms the favourable uptake of the layered double hydroxide adsorption process.
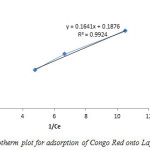 |
Figure6: Langmuir Isotherm plot for adsorption of Congo Red onto Layered Double Hydroxide Click here to View figure |
From the plot of Inqe against InCe the Freundlich constants Kf and n which respectively indicating the adsorption capacity and the adsorption intensity, were calculated from the intercept and slope as shown in figure 6 and table 1.
The fraction of the layered double hydroxide surface covered by the Congo Red is given as 0.47 (table 1). This value indicates that 47% of the pore spaces of the Layered double hydroxide surface were covered by the Congo Red which means high degree of adsorption.
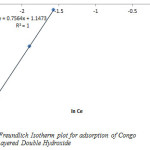 |
Figure7: Freundlich Isotherm plot for adsorption of Congo Red onto Layered Double Hydroxide Click here to View figure |
The plot of lnqe against e2 is shown in Figure 8 and the constants qD and BD were calculated from the intercept and slope respectively. The DKR constants such as qD, BD, and the apparent energy E where calculated to be 0.9589, 1.0635 and 0.6859KJ/mol respectively. If the value of E lies between 8 and 16kJ/mol the sorption process is a chemisorptions one, while values of below 8kJ/mol indicates a physical adsorption process. The value of the apparent energy of adsorption (0.6859kJ/mol) obtained indicated physiosorptions between layered double hydroxide and Congo Red dye.
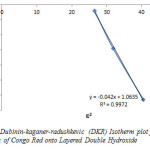 |
Figure8: Dubinin-kaganer-radushkevic (DKR) Isotherm plot for adsorption of Congo Red onto Layered Double Hydroxide Click here to View figure |
Temkin adsorption isotherm model is usually chosen to evaluate the adsorption potentials of an adsorbent for the adsorbate from an experimental data. This model gives the mechanism and adsorption capacity of an adsorbate in a sorption process. By plotting qe against Ince, the Temkin constants A and B where calculated from the slope and intercept. The constants A and B are 1.377 and 2.0498 respectively while the correlation coefficient value was 0.9689, indicating that the adsorption process is physical.
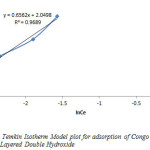 |
Figure9: Temkin Isotherm Model plot for adsorption of Congo Red onto Layered Double Hydroxide Click here to View figure |
Table1: Characteristic Parameters of the Adsorption Isotherm Models for Congo Red adsorption by Layered Double Hydroxide
|
Isotherm Model |
Isotherm Parameter |
Results |
| Freundlich | 1/n | 0.872 |
| Kf | 2.1306 | |
| R2 | 1 | |
| Langmuir | Sf, | 0.7 |
| R2 | 0.9924 | |
| Dubinin- kaganer-Radushkevich | E, KJ/mol | 0.6859 |
| bD, mol2/KJ2 | 1.0635 | |
| qD, mg/g | 0.9589 | |
| R2 | 0.9972 | |
| Temkin | A | 1.377 |
| b | 1.107×103 | |
| B | 2.0498 | |
| R2 | 0.9689 |
Effect of Temperature
As shown in figure 10 adsorption was lowest at 313K (51%), and increased slightly to 333K (53%) and 353K (55.5%). This means that adsorption capacity increase with higher temperature.
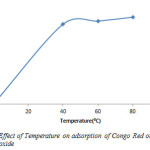 |
Figure10: Effect of Temperature on adsorption of Congo Red onto layered double hydroxide Click here to View figure |
The values of the enthalpy change (∆Ho) and entropy change ∆So were calculated from equation 10 to be 4.152KJ/mol and 13.5J/molK respectively, as shown in figure 11. A positive ∆Ho suggests that sorption proceeded favourably at higher temperature and the sorption mechanism was endothermic. A positive value of ΔS0 (13.5J/molK) reflects the affinity of the adsorbent towards the adsorbate species. In addition, positive value of ΔS0 suggests increased randomness at the solid/solution interface with some structural changes in the adsorbate and the adsorbent. The adsorbed solvent molecules, which are displaced by the adsorbate species, gain more translational entropy than is lost by the adsorbate ions/molecules, thus allowing for the prevalence of randomness in the system [23, 24, 25]. The positive ΔS0 value also corresponds to an increase in the degree of freedom of the adsorbed species.
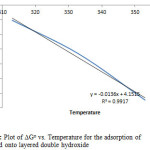 |
Figure11: Plot of DGo vs. Temperature for the adsorption of Congo Red onto layered double hydroxide Click here to View figure |
Isosteric heat of adsorption DHx is one of the basic requirements for the characterization and optimization of an adsorption process and is a critical design variable in estimating the performance of an adsorptive separation process. It also gives some indication about the surface energetic heterogeneity. Knowledge of the heats of sorption is very important for equipment and process design. A plot of InCe against 1/T in figure 12 gives a slope equal to ΔHx. The value of ΔHx derived from equation 11 was 39.9KJ/mol which indicates that adsorption mechanism was physical adsorption and in an heterogeneous surface [24, 25].
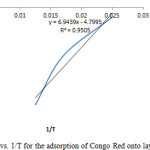 |
Figure12: Plot of In Ce vs. 1/T for the adsorption of Congo Red onto layered double hydroxide Click here to View figure |
The activation energy Ea and the sticking probability S* were calculated from equation 12, the value shown in table 1 for Ea and S* are -9.34KJ/mol and 0.49 respectively, as shown in the plot in figure 13. The value of activation energy shows that the sorption process was a physical one less than 4.2KJ/mol. The sticking probability S* indicates the measure of the potential of an adsorbate to remain on the adsorbent. It is often interpreted as S*>1 (no sorption), S*=1 (mixture of physic-sorption and chemisorption), S* = 0 (indefinite sticking – chemisorption), 0<S*<1 (favourable sticking – physic- sorption) [25].
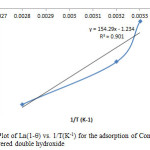 |
Figure13: Plot of Ln(1-q) vs. 1/T(K-1) for the adsorption of Congo Red onto layered double hydroxide Click here to View figure |
Table2: Thermodynamic Parameters of the Adsorption of Congo Red onto Layered Double Hydroxide
| T, K | ΔGo, KJ/mol | ΔHo, KJ/mol | ΔSo, J/molK | Ea KJ/mol | ΔHx KJ/mol |
|
313 |
-0.109 |
4.152 |
13.5 |
-9.34 |
39.9 |
|
333 |
-0.332 |
||||
|
353 |
-0.646 |
Effect of Time
The adsorption kinetic study is important in predicting the mechanisms (chemical reaction or mass-transport process) that control the rate of the pollutant removal and retention time of adsorbed species at the solid-liquid interface. That information is important in the design of appropriate sorption treatment plants.
The effect of contact time of the phases on removal of Congo Red by the Layered double hydroxide from solutions of initial concentration equal to 400mg CR/L at three different times (10, 20 and 30 minutes) is presented in Figure 14.
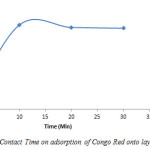 |
Figure14: Effect of Contact Time on adsorption of Congo Red onto layered double hydroxide Click here to View figure |
The result shows that adsorption was highest at 10 minutes, thereafter, a gradual decrease occurred (10=52%, 20=50.5% and 30=50%).
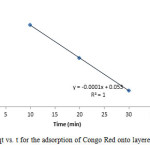 |
Figure15: Plot of qt vs. t for the adsorption of Congo Red onto layered double hydroxide Click here to View figure |
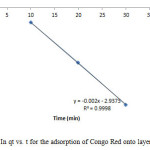 |
Figure16: Plot of In qt vs. t for the adsorption of Congo Red onto layered double hydroxide Click here to View figure |
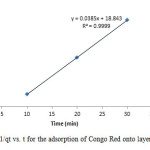 |
Figure17: Plot of 1/qt vs. t for the adsorption of Congo Red onto layered double hydroxide Click here to View figure |
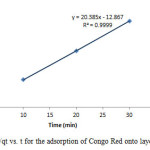 |
Figure18: Plot of 1/qt vs. t for the adsorption of Congo Red onto layered double hydroxide Click here to View figure |
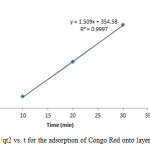 |
Figure19: Plot of 1/qt2 vs. t for the adsorption of Congo Red onto layered double hydroxide Click here to View figure |
The experimental data were fitted into different kinetic models including (Figures 15 – 19) zero-order-kinetic model, first-order-kinetic mode, second-order-kinetic model, pseudo-second-order-kinetic model and third-order-kinetic model to ascertain the suitability of the models. The correlation coefficient values of 1, 0.9998, 0.999, 0.9999 and 0.9997 respectively confirms the applicability of the chosen kinetic models.
Conclusion
The present investigation shows that Ni/Al-CO3 synthesized by coprecipitation method can be employed as a potentially viable sorbent for the removal of Congo Red dye from industrial wastewaters. The Congo Red adsorption was found to be greatly dependent on temperature. The experimental data were well defined by Langmuir, Freundlich, Temkin and Dubinin- kaganer-Radushkevich isotherms. The experimental data also fitted all the kinetic models applied in this paper. The values of ΔHo and ΔSo indicated that the adsorption process was endothermic and process is dependent on increase in temperature, thereby increasing the randomness of the solid/liquid phase of the reaction system.
References
- Zhiqiao, H. Shuang, S. Huamin, Z. Haiping, Y. and Jianmeng, C., C.I.Reactive Black 5 decolorization by combined sonolysis and ozonation., Ultrasonics Sonachem., 2007., 14 (3), 298- 304
- Sharma, J. and Janveja, B. A study on removal of congo red dye from the effluents of textile industry using rice husk carbon activated by steam, Rasayan J. Chem., 2008, 1(4), 936-942.
- Shen, D., Fan, J., Zhou, W., Gao, B., Yue, Q. & Kang, Q.. Adsorption kinetics and isotherm of anionic dyes onto organo-bentomite from single and multisolute systems. J. Hazard. Mater. 2009.,172, 99-107.
- Kyzioł-Komosińska, J., Rosik-Dulewska, C., Pająk, M. & Jarzyna, M.. Removal of direct dyes from wastewater by sorption onto smectite clay. Arch. Environ. Prot. 2010.,3, 3–14.
- Bhattacharyya K.G. and Sharma, A. “Kinetics and Thermodynamics of Methylene Blue Adsorption on Neem Leaf Powder” Dyes and Pigments, (2005), 65, 51-59.
- Mall, I.D., Srivastava, V.C., Agarwal, N.K.and Mishra , I.M., “Adsorptive Removal of Malachite Green Dye from Aqueous Solution by Bagasse Fly Ash and Activated Carbon – Kinetic Study and Equilibrium Isotherm Anal- yses”, Colloids and Surfaces: A physicochemical. Eng. Aspects, 2005., 264, 17-28.
- Amin, S., Jayson, G.G.,. Humic substance uptake by hydrotalcites and PILCs. Water Res. 1996., 30, 299 – 977.
- Cavani, F., Trifiro, F., Vaccari, A., Hydrotalcite-type anionic clays: preparation, properties and application. Catal. Today 1991., 11, 173 – 301.
- Fetter, G., Ramos, E., Olguin, M.T., Bosh, P., Lopez, T., Bulbulian, S., Sorption of 131I by hydrotalcites. J. Radioanal. Nucl. Chem. 1997. 222, 63 – 66.
- Gutmann, N.H., Spiccia, L., Turney, T.W.,. Complexation of Cu(II) and Ni(II) by nitriloacetate intercalated in Zn – Cr layered double hydroxides. J. Mater. Chem. 2000.,10, 1219 – 1224.
- Hermosín, M.C., Pavlovic, I., Ulibarri, M.A., Cornejo, J., Trichlorophenol adsorption on layered double hydroxide: a potential sorbent. J. Environ. Sci. Health, Part A, Environ. Sci. Eng. 1993., 28 (9), 1875 – 1888.
- Hermosín, M.C., Pavlovic, I., Ulibarri, M.A., Cornejo, J.,. Hydrotalcite as sorbent for trinitrophenol: sorption capacity and mechanism. Water Res. 1996 30, 171 – 177.
- Houri, B., Legrouri, A., Barroug, A., Forano, C., Besse, J.P., Use of the ion exchange properties of layered double hydroxides for water purification. (1998).
- J. Tronto, K.C. Sanchez, E.L. Crepaldi, Z. Naal, I.K. Stanlei, J.B. Valim Synthesis, characterization and electrochemical stidy of layered double hydroxide intercalated with 2thiophenecarboxylate anions, J. Phys. Chem. Solid (2004), 65 493-498.
- M.Z. Hussein, A.H. Yahaya, Z. Zainal, L.H. Kian, Nanocomposite-based controlled release formulation of an herbicide, 2, 4-dichlorophenoxyacetate incapsulated in zinc-aluminiumlayered double hydroxide, Sci. Technol. Adv. Mat. 2005.,6., 956-962.
- Pavlovic, I., Ulibarri, M.C., Hermosín, M.C., Cornejo, J.,. Sorption of an anionic surfactant from water by a calcined hydrotalcite-like sorbent. Fresenius Environ. Bull. 1997., 6, 266 – 271.
- Pavlovic, I., Barriga, C., Hermosín, M.C., Cornejo, J., Ulibarri, M.C., Adsorption of acidic pesticides 2,4-D, Clopyralid and Picloram on calcined hydrotalcite. Appl. Clay Sci. 2005., 30, 125 – 133.
- Rives, V., Ulibarri, M.A.,. Layered double hydroxides (LDH) intercalated with metal coordination compounds and oxometalates. Coord. Chem. Rev. 1999 181, 61 – 120.
- Tarasov, K.A., O’Hare, D., Isupov, V.P., Solid state chelation of metal ions by ethylenediaminetetraacetate intercalated in a layer double hydroxide. Inorg. Chem. 2003. 42 (6), 1919 – 1927.
- Tsyganok, A.I., Suzuki, K., Hamakawa, S., Takehira, K., Hayakawa, T., Mg – Al layered double hydroxide intercalated with [Ni (edta)] 2 − chelate as a precursor for an efficient catalyst of methane reforming with carbon dioxide. Catal. Letters 2001. 77 (1–3), 75 – 86.
- Ayawei, N., Ekubo, A. T., Wankasi, D., and Dikio, E. D. Mg/Fe Layered Double Hydroxide For Removal Of Lead (Ii): Thermodynamic, Equilibrium And Kinetic Studies. European Journal of Science and Engineering. 2015. 3,.1; 1-17.
- Ayawei, N., Ekubo, A. T., Wankasi, D., and Dikio, E. D.. Equilibrium, Thermodynamic and Kinetic Studies of the Adsorption of Lead(II) on Ni/Fe Layered Double Hydroxide. Asian Journal of Applied Sciences .,2015., 3 – Issue 2; 2.,7-217.
- Ayawei, N., Ekubo, A. T., Wankasi, D., and Dikio, E. D. “Synthesis and Application of Layered Double Hydroxide for the removal of Copper in Wastewater”. International Journal of Chemistry. 2015.,. 7., 1; 122 – 132.
- a) Arivoli S, Venkatraman B R, Rajachandrasekar T and Hema M, Adsorption of ferrous ion from aqueous solution by low cost activated carbon obtained from natural plant material, Res J Chem Environ. 2007.,17, 70-78. b)
- Dawodu F. A., Akpomie G. K., and Ogbu I. C., “Isotherm Modeling on the Equilibrium Sorption of Cadmium (II) from Solution by Agbani Clay”, International Journal of Multidisciplinary Sciences and Engineering, 2012. 3, 9.
- K.K.H. Choy, G.Mckay and J.F. Porter “Sorption of acidic dyes from effluents using activated carbons”, Resource. Conserv. Recycling, 1999,.27., 57-71.
- Chongrak, K., Eric, H., Noureddine A. and Jean P. G., “Application of Methylene Blue Adsorption to Cotton Fiber Specific Surface Area Measurement: Part I. Methodology”, The Journal of Cotton Science 1998., 2: 164 – 173.

This work is licensed under a Creative Commons Attribution 4.0 International License.









