Urea biosensor based on immobilization of urease on ZnO nanoparticles
Mostapha Eghbali1, Afshin Farahbakhsh1, Aliasghar Rohani2 and Ali Nakhaei Pour3
1Islamic Azad University - Quchan Branch, Iran Quchan. 2Research Institute of Industrial Petroleum, Tehran, Iran. 3Department of Chemistry, Ferdowsi university of Mashhad, P.O.Box: 9177948974, Mashhad, Iran
DOI : http://dx.doi.org/10.13005/ojc/310284
Article Received on :
Article Accepted on :
Article Published : 28 Apr 2015
The properties of immobilized urease enzyme on nanostructured zinc oxide particles were studied at various conditions. Zinc oxide thick films were deposited on Au-sheet using organic additives and urease enzyme is immobilized at various pH (6, 7 and 8). The activity of produced biosensors was evaluated at various concentrations of urea (10-6, 10-7 and 10-8 M). The result show that when pH increased from 6.0 to 8.0, the biosensor shows an optimal response at pH=7. Also, the response of biosensor in pH=6 is lower than pH=8. With the increasing of urea concentration, the response of produced biosensor is increased and this trend was the same for three pH of solution. The results show that at optimum condition (pH=7, 10-6M), for the imprinted ZnO films on Au maximum current is 18.4 mA, while the maximum current for immobilized urease on Au sheets without ZnO films is 6.06 mA.
KEYWORDS:Nano structured zinc oxide; Enzyme immobilization; Urea biosensor
Download this article as:| Copy the following to cite this article: Eghbali M, Farahbakhsh A, Rohani A, Pour A. N. Urea biosensor based on immobilization of urease on ZnO nanoparticles. Orient J Chem 2015;31(2). |
| Copy the following to cite this URL: Eghbali M, Farahbakhsh A, Rohani A, Pour A. N. Urea biosensor based on immobilization of urease on ZnO nanoparticles. Orient J Chem 2015;31(2). Available from: http://www.orientjchem.org/?p=8642 |
Introduction
Urea (also known as carbamide) is a waste product of many living organisms, and is the major organic component of human urine. This is because it is at the end of chain of reactions that break down the amino acids that make up proteins. Urea analysis is of considerable interest in clinical and agricultural chemistry [1-2]. Urea estimation is important during environmental monitoring and many methods are available for urea estimation [1, 3]. However, these methods suffer from complicated sample pretreatment steps and are unsuitable for on-site monitoring. It is envisaged that biosensors can be utilized to overcome some of these problems. A biosensor is an analytical device based on the direct spatial coupling of an immobilized biologically active compound with a compatible transducer, which converts the biochemical signal into a quantifiable electrical signal [4-5].
Enzyme immobilization is an important aspect for the development of biosensors and bioreactors for new aspect of biotechnology [6-7]. Immobilization of enzyme on a suitable support is a common requirement to achieve reusable catalyst and to improve economics. Immobilized enzymes have many advantages such as good thermal and operational stability, reusability, continuous use and easy separation of the enzyme from product. The main disadvantage of the immobilized enzymes is the possible loss of enzyme activity during immobilization. The immobilization studies of enzyme focus on the material selection, methods and characters of enzyme [7-9]. Many methods have been developed for the enzyme immobilization but usually one of four methods is used: physical adsorption, entrapment, co-polymerization and covalent at attachment [10-12].
In recent years, nanomaterials such as metal nanoparticles and semiconducting nanoparticles are receiving considerable in enzyme immobilization process. These interests are owing to unique structure, high chemical stability and high surface-to-volume ratio of nanomaterials. These properties make them extremely attractive for fabricating chemical sensors [10, 13-14].
In the present study, the urease was immobilized onto ultrathin ZnO films at various pH of buffer and a cheap and miniaturized urea biosensor was developed. The effect of pH on immobilization of urease and the activity of produced biosensor was studied.
Experimental
Materials
Urease enzyme (EC 3.5.1.5, from jack bean, 50 U/mg), zinc nitrate hexahydrate (Zn(NO3)2, 6H2O) and Hexamethylenediamine (H2N(CH2)6NH2 ) were purchased from Sigma Aldrich company. Urea (Mw= 60.06 gr/mol), Isopropanol (98%) and Deionized (DI) water (resistivity of 18 MΩ cm) was obtained from Iranian companies. Urea and other chemicals were of analytical grade and used without further purification.
Thick Film Preparation of ZnO Nanoparticles
The solution method was used for synthesis of zinc oxide nanostructured particles (see Fig.1). For this propose a solution of zinc nitrate hexahydrate (0.2-0.3 molar, M) was mixed with a solution of Hexamethylenediamine (0.10-0.12 M), stirred well and refluxed at 90 oC for 1 h each. This solution was applied for deposition of zinc oxide nanoparticles on Au-sheet (5 cm×0.3 cm). Before transfer into the solution, the Au-sheet was polished with acetone and isopropanol carefully. Then the Au-sheet was transfer into solution and thick film of ZnO was allowed to settle at 90 oC during the past 15 minutes. The Au-sheet was removed from the solution and dried at 100 °C for 24 h.
Characterization of ZnO Film
Morphological observations of powder and thick film samples were carried out by field emission scanning electron microscopy (SEM, FESEM, Hitachi S4700).
Elemental and compositional analysis was carried out using X-ray diffraction (XRD) spectrometry. The XRD measurements were performed using a Bruker axs Company, D8 Advance diffractometer (Germany) using monochromatized Cu/Kα radiation (40 kV, 40 mA) and a step scan mode at a scan rate of 0.02° (2θ) per second. Specimens for XRD were prepared by compaction into a glass-backed aluminum sample holder. Data were collected over a 2θ range from 30-80° and phases were identified by matching experimental patterns to entries in the Diffracplus Version 6.0 indexing software. XRD peak identification was performed by comparison with the JCPDS database.
Enzyme Immobilization
Urease was immobilized by soaking the samples in urease solution. Initially samples were immersed in a phosphate buffer solution (PBS, 0.1M) containing 0.05 g of urease per ml for 20 min at 25 oC. The pH of buffer solution was changed from 6 to 8, for evaluation of pH effects in urease immobilization process. The samples were then washed and kept in PBS until use. Amount of enzyme immobilized on these samples was not calculated or estimated.
Determination of Enzyme Activity After Immobilization
After urease immobilization, the electrical property of ZnO thick films was studied to determine the enzyme activity. A cell was constructed consisting of Ag/AgCl electrode and urease immobilized ZnO films were used as another electrode (Fig.1). Urea solution prepared in PBS was used as an electrolyte. Amount of electrolyte was kept constant as 10 ml for all measurement. Solution was prepared with various concentrations of urea (10-6, 10-7 and 10-8 M). For this solution preparation, the amount of PBS was kept constant as 10 ml while the molar concentration of urea was calculated with reference to 10 ml of PBS. The respective amount of urea was added in separate beakers. Since the amount of urea with respect to PBS has changed with increasing molar concentration, this effect would reflect in the sensitivity curves indicating the variation due to urea, as presented below. The ratio of current to voltage is used as a measure of enzyme activity.
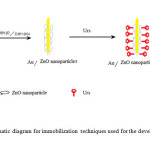 |
Figure1: Schematic diagram for immobilization techniques used for the development of urea biosensor. Click here to View figure |
Electrochemical properties were studied with AMEL Potentiostat (7050) at room temperature in a conventional three-electrode system. ZnO thick films were used as working electrode, platinum wire (1mm diameter) was used as auxiliary electrode and Ag/AgCl as a reference electrode. Solution with various urea concentrations was used as electrolyte.
Results and Discussion
Characterization of ZnO Film
The characteristic morphology of the as-synthesized ZnO film can be observed by SEM micrograph. Typical SEM images are shown in Fig.2. SEM observation indicates that the obtained ZnO film is dense, rough, and plentiful holes are present at the surface of the film.
 |
Figure2: SEM image of ZnO film Click here to View figure |
The XRD of the as-grown ZnO micro-pompons is shown in Fig.3. All the diffraction peaks can be indexed as the hexagonal wurtzite structure ZnO in the standard data (JCPDS, 36-1451) [15].
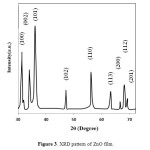 |
Figure3: XRD pattern of ZnO film. Click here to View figure |
The sharpness of the peaks indicates that the products are well crystallized. No diffraction peaks for impurities are observed in the pattern.
Application as Urea Biosensor
Evaluation of pH Effects in Urease Immobilization Process
The pH has a significant effect on immobilization of urease and the activity of produced biosensor is affected by it. Enzymatic urea detection is generally based on determining the products of urea based hydrolysis formed in accordance with Eq. (1):
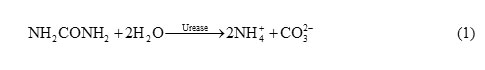
This reaction results in the production of three ions (i.e. two NH4+ and CO32−) from uncharged urea which increases the conductivity of the host material by providing excess electron to the conduction band of the material. The ammonium ions react with the ZnO and produce the corresponding electromotive force (EMF) change of the output of the sensor, therefore the electrochemical characterization of the biosensor is studied by cyclic voltammetry (CV).
The pH of buffer solution in urease immobilization process was changed from 6 to 8 on nanostructured ZnO film in 10-6 M solution. Fig.4 shows typical CVs of urease/ZnO /Au electrode with scan rate of 20 mV/s for urea biosensor prepared at pH=6, 7 and 8. The effect of pH on the urease/ZnO/Au biosensor was investigated by measuring the reduction peak current. As shown in Fig.4, with pH increasing from 6.0 to 8.0, the biosensor shows an optimal response at pH=7. In addition, it was found in low pH (pH=6) the response of biosensor is lower than high pH levels (pH=8) of the immobilization process.
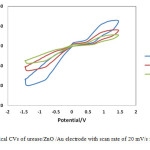 |
Figure4: Typical CVs of urease/ZnO /Au electrode with scan rate of 20 mV/s for various pH.
|
Evaluation of Substrate Concentration on Urease Immobilized Responses
It is well known that enzymatic activity of immobilized biosensor depends on the substrate concentration. Thus the concentrations of urea in solution was changed from 10-6, 10-7 and 10-8 M for three immobilized biosensors at pH=6, 7, 8. Fig.5 shows CVs of urease/ZnO /Au electrode with scan rate of 20 mV/s for immobilized biosensors at pH=6, 7 and 8 and various concentrations of urea (10-6, 10-7 and 10-8 M). As can be seen, with the increasing of urea concentration, the response of produced biosensor is increased and this trend was the same for three pH of solution. At low urea concentrations, due to lack of substrate, enzyme active sites is not yet fully complete and immobilized enzyme activity was low. With increasing substrate concentration, enzyme activity increases until all positions are filled by active enzymes. In this condition the enzyme activity is reached to maximum and the activity of sensor does not affected by enzyme concentration of solution. As shown in Fig.5, the biosensor response is increased with increasing of urea concentration and reduced when the pH is far from neutral pH range. Also this Fig shows that the current pick is produced around the 0.85 volt and this voltage is optimum for sensor activity.
 |
Figure5: Typical CVs of urease/ZnO /Au electrode with scan rate of 20 mV/s for various concentrations of urea (10-6, 10-7 and 10-8 M) (a) pH=8, (b) pH=7, (c) pH=6. Click here to View figure |
Fig. 6 shows the effects of concentration of urea on immobilized biosensor response at various concentrations at 0.85 volt. With the increasing urea concentration, a greater current response was observed for the imprinted ZnO films (Fig. 6) for all biosensores. As shown in Fig.6, at optimum condition (pH=7, 10-6M), maximum current is 18.4 mA for the imprinted ZnO films on Au. While the maximum current for immobilized ureas on Au sheets without ZnO films is 6.06 mA. This result shows that the coating of Au sheets with ZnO nanoparticles, increased the available surface in immobilization process.
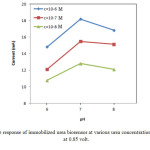 |
Figure6: The response of immobilized urea biosensor at various urea concentration and buffer pH at 0.85 volt. Click here to View figure |
Conclusions
A feasibility study is presented for the use of nanostructured zincoxide in the form of a thick film for urea sensing at room temperature. Zinc oxide thick films were deposited on Au-sheet using organic additives and urease enzyme is immobilized at various pH (6, 7 and 8). By coating of Au sheets with ZnO nanoparticles, the available surface for immobilization of urease enzyme is increased and the maximum current for immobilized ureas be tripled. With increasing of urea concentration, the response of produced biosensor is increased and this trend was the same for three pH of solution. At optimum condition (pH=7, 10-6M), maximum current is 18.4 mA for the imprinted ZnO films on Au.
References
- Devi, R.; Pundir, C.S. Sens. Actuators, B. 2014, 193, 608-615
- Ahmad, R.; Tripathy, N.; Hahn, Y. B. Sens. Actuators, B. 2014, 194, 290-295
- Raghu, P.; Madhusudana Reddy, T.; Reddaiah, K.; Kumara Swamy, B.E.; Sreedhar, M. Food Chem. 2014, 142, 188-196
- Yi, Z.; Wang, H. B.; Chen, K.; Gao, Q.; Tang, H.; Yu, R. Q.; Chu, X. Biosens. Bioelectron. 2014, 53, 310-315
- Das, A.P.; Kumar, P.S.; Swain, S. Biosens. Bioelectron. 2014, 51, 62-75
- Sassolas, A.; Blum, L.J.; Leca-Bouvier, B.D. Biotechnol. Adv. 2012, 30, 489-511
- Ansari, S.A.; Husain, Q. Biotechnol. Adv. 2012, 30, 512-523
- Arya, S.K.; Saha, S.; Ramirez-Vick, J.E.; Gupta, V.; Bhansali, S.; Singh, S.P. Anal. Chim. Acta. 2012, 737, 1-21
- Dhawan, G.; Sumana, G.; Malhotra, B.D. Biochem. Eng. J. 2009, 44, 42-52
- Ansari, S.G.; Wahab, R.; Ansari, Z.A.; Kim, Y. S.; Khang, G.; Al-Hajry, A.; Shin, H. S. Sens. Actuators. B. 2009, 137, 566-573
- Kamarchuk, G.; Pospelov, A.; Kushch, I. in: Amann, A.; Smith D. (Eds.), Volatile Biomarkers, Elsevier, Boston, 2013, 264-300
- Chen, X.; Yang, Z.; Si, S. J. Electroanal. Chem. 2009, 635, 1-6
- Ali, S.M.U.; Ibupoto, Z.H.; Salman,S.; Nur, O.; Willander, M.; Danielsson,B.; Sens. S. Actuators, B. 2011, 160, 637-643
- Ansari, S.G.; Ansari, Z.A.; Seo, H. K.; Kim, G. S.; Kim, Y. S.; Khang, G.; Shin, H. S. Sens. Actuators, B. 2008, 132, 265-271
- Zhou, Y.; Wang, L.; Ye, Z.; Zhao, M.; Huang, J. Electrochim. Acta. 2014, 115, 277-282

This work is licensed under a Creative Commons Attribution 4.0 International License.









