The influence of Neutron Irradiation in CR-39 polymer
Sangeeta Prasher1, Mukesh Kumar2* and Surinder Singh3
1Department of physics, Kanya Maha Vidayalaya, Jalandhar-144004, India.
2Department of Physics, Lovely Professional University, Phagwara-144411, India.
3Department of Physics, Guru Nanak Dev University, Amritsar-143005, India.
Corresponding Author: (moka.esh9@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/310277
Article Received on :
Article Accepted on :
Article Published : 29 May 2015
The script allocates the influence of neutron irradiations on the structural and optical properties of CR-39. The structural properties of the polymer have been examined using the FTIR spectrum of the pristine and neutron beam irradiated polymer. The studies reveal the increase the intensity of some bands with neutron irradiation pointing the increase in the unsaturated behavior of the polymer. The optical properties analyzed using the UV-Vis spectra made it evident that CR-39 gets easily influenced at a fluence of 1016 n/cm2. The glassy characteristics of the polymer also found to diminish with increasing neutron irradiation. Significant variations in the property profile of the polymer have been observed at higher neutron fluence.
KEYWORDS:Irradiation; FT-IR; Optical Properties; UV-Vis spectroscopy
Download this article as:| Copy the following to cite this article: Prasher S, Kumar M, Singh S. The influence of Neutron Irradiation in CR-39 polymer. Orient J Chem 2015;31(2). |
| Copy the following to cite this URL: Prasher S, Kumar M, Singh S. The influence of Neutron Irradiation in CR-39 polymer. Available from: http://www.orientjchem.org/?p=8899 |
Introduction
The operation of radiations on polymer composites is of great importance with a view to achieve some desired improvements in polymer properties1-4 that cannot be achieved by chemical courses. It is also worthwhile to study the modifications in the structure and optical properties of polymer due to irradiation. However, the effectiveness of these changes produced depends on the structural conformation of the polymer as well as the experimental conditions of irradiation5-6.
Since the introduction of CR-39 as a solid state nuclear track detector7-8, many authors have shown keen interest to use CR-39 in neutron dosimetry9-11 and neutron detection12-13. But so far a very little attention has been paid to study the influence of neutron irradiation on the structural, optical and etching properties of the polymer14-15. The behavior of neutrons in the matter is different to that of alpha and beta particles as they don’t carry any charge and exerts no columbic forces on either orbital electrons or the nuclei. Hence forth, neutrons to affect matter, they must either strike the nuclei or come close enough to interact with nuclear forces.
The present investigation have been made to study the influence of neutron irradiation on optical and structural properties of CR-39 and to study the variation in optical response of the polymer with neutron fluence so as to facilitate neutron dosimetry with an easy and less time consuming process.
Experimental Technique
Pieces of CR-39 of 230mm procured from Pershore Mouldings Ltd were washed, dried and packed in polyethylene sachet of 1x3cm2 and sent to BARC, Mumbai. The irradiation has been carried out in the thermal column of APSARA at a flux of 1011neutrons/cm2/sec. The samples were exposed to different fluence ranging between 1014 to 1018 neutrons/cm2. UV-VIS and FTIR spectral studies of the pristine and neutron irradiated polymer have been made with the help of Shimadzu 1601 UV-VIS spectrophotometer and Perkin Elmer 8000 FTIR Spectrophotometer, respectively.
Results and Discussion
The color transformations along with the morphological changes have been observed in CR-39 with the increase of neutron fluence. The plasticity of the polymer has been found to increase with neutron fluence that can be attributed to the changes in the tensile strength of the polymer. It can also be ascribed to the formation of the low-molecular weight degradation products which acts as plasticizers for the polymer16. The polymer originally transparent and glassy got the brownish tinge on neutron irradiation that may be due the trapping of free radicals and ions. The presence of trace amount of impurities and additives may further enhance discoloration17-18 have also reported the color transformation of CR-39 in case of γ-irradiation of the polymer.
FTIR and UV-VIS spectral studies of the pristine and neutron irradiated CR-39 have been made to study the structural and optical changes to analyze the reaction mechanism causing such a great changes in the morphology of the polymer and are discussed.
FTIR Spectral Analysis
The structural changes induced in the polymer have been analyzed by the relative changes in the band intensities of the functional groups present in the monomeric unit of the polymer (fig. 1). A strong absorption band from carbonate ester bond (>C=O) at 1725cm-1 along with some other bands at 3542, 2984, 2368 and 1650 cm-1 corresponding to free –OH, methyl or methylene stretching , CO2 and double bonded carbons conjugated to carbonyl groups respectively have been observed for pristine CR-39. The peaks at 1581 and 2214cm-1 notify the presence of [-(C=O)-C=C] and CºC species indicating the partial polymerization of the monomer. The intensity of the bands has been found to increase with neutron fluence pointing the increase in the unsaturated behavior of the polymer. The band corresponding to carboxylate ions found to disappear with increasing neutron fluence. The increase in the intensity of the band corresponding to CO2 and the decrease in the intensity of >C=O band have also been observed. It may be due to the cleavage of chain at carbonate linkage causing the production of CO2 and carboxylic acids. Thus this linkage may be assumed as radiation sensitive linkage. Chung19 has also reported the degradation of the gamma irradiated polycarbonates at carbonate linkage and evidences the release of CO2. The broadening of >C=O band with neutron irradiation has been observed and may be due to the inter-hydrogen bonding. Shift of –OH band towards lower frequencies also evidences the hydrogen bonding and the bondage of free –OH group to other free radicals and ions. The increase in the intensity of the –OH band may attributed to the abstraction of –H atom from the back bone of the polymeric unit and the formation of alcohols and carboxylic acids. The increase in the intensity of CºC also points out the knock out of hydrogen atoms from the polymeric chains by neutron collision. The generation of CO2 and –OH group has been also been observed and reported by Malek and Chong20 and Yamauchi et al.21 during the γ-rays exposure of CR-39. The constancy in the intensity of C-O-C stretch indicates the stable nature of chain around that linkage.
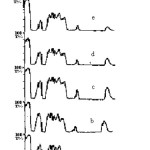 |
Figure1: FTIR spectra of pristine (a) and neutron exposed (b-f) CR-39. Click here to View figure |
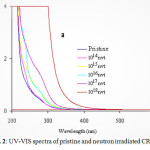 |
Figure2: UV-VIS spectra of pristine and neutron irradiated CR-39 Click here to View figure |
UV-VIS Spectral Analysis
UV-VIS spectral studies are helpful in determining the direct band gap energy of the polymers. The UV-VIS spectra of pristine neutron irradiated CR-39 is shown in fig. 2. It is evident from the figure that the absorption edge is shifting towards longer wavelength with increasing neutron fluence.
The band gap and Urbach’s energies of the pristine and neutron irradiated polymers have been calculated and are reported in table 1. The band gap energy of the polymer has been found to decrease with neutron fluence which indicates the decrease in the resistivity of the polymer. The authors22 have already reported the decrease in the resistivity of CR-39 and Makrofol-KG with ion irradiation. Sonkawade et al.23 have also reported improvement in the crystallinity of pani with gamma and neutron irradiation. The decrease may be attributed to the production of free radicals and hot molecules.
The study of Urbach’s tail, the exponential band tail in non-crystalline materials is important in understanding the electronic transport phenomenon in these materials. The electronic density of states of amorphous solid is altered from that of the crystalline solid with broadened peaks and decays into the optical band gap of the solids and originates from the strains in the network sufficient to push states past the band edges into the gap. The energy associated with such diffusion is termed as Urbach’s energy and is used to study disordering processes in solids. The Urbach rule contributes to important information about dynamic properties of elementary excitations in materials. The Urbach’s energy of CR-39 has been found to decrease with neutron fluence (Table 1), indicating the decrease in the strain of the network of the polymer and the reduced length of disorder in the forbidden band. The reduced energy concluded the reduction in hydrogen evolution in the polymer matrix and its bondage to some free radicals. The variations in the tensile strength of the LDPE due to weather conditions have also been discussed by Tuasikal et al.24.
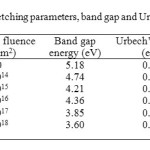 |
Table1: The variation in etching parameters, band gap and Urbach’s energy with neutron fluence in case of CR-39 Click here to View table |
Conclusions
The polymer is found to get influenced by the neutron irradiation at higher fluence. The variations in the properties are first evident from the color transformations of CR-39. The band gap of the polymer have been found to associate with the π→π* transitions and the variations may have been induced due to the production of free radicals in the polymer matrix, that cause transitions associated with π→n states.
References
- Mishra, R.; Tripathi, S.P.; Sinha, D.; Dwivedi, K.K.; Ghosh, S.; Khathing, D. Huller, M.; Fink, D. Chung, W. Nucl. Instrum. Meth. B 2000, 168, 59-64
- Mishra, R.; Tripathy, S.P.; Dwivedi, K.K.; Khathing, D.T.; Ghosh, S.; Muller, M.;Fink, D.; Radiat. Meas. 2003, 36, 621.
- Singh, P.; Kumar, R. Vacuum, 2013, 96, 46-51.
- Prasher, S.; Kumar, M.; Singh, S. Inter. J. Polym. Anal. & Character. 2014, 19, 204.
- Dole, M.; The radiation Chemistry of Macromolecules. Academic Press, New York 1972, 1.
- Singh, P.; Kumar, R.; Prasad, R. Radiat. Eff. Defects Solids, 2013, 168: 97.
- Cartwright, B.G., Shirk E.K. and Price, P.B. Nucl. Instrum. Meth. 1978, 153, 451.
- Casson, R.M., and Benton, E.V. Nucl. Track Detect. 1978, 2, 173-179.
- Decossas, J.L. and Vareille, J.C. Radiat. Ptotect. Dosim. 1987, 20(1-2), 41.
- Matiullah; Kudo, K.; Khan, S. and Khan, H.A., Nucl. Track Radiat. Meas. 1991, 90, 505.
- Meyer, P.; Groetz, J.E.; Fromm, M.; Lacourt, A. and Chambande, A. Radiat. Meas. 1997, 28, 423.
- Vilela, E.; Fantuzzi, E.; Giuacomelli, G. Radiat. Meas. 1991, 31, 437.
- Spurny, F.; Turek, K. Nucl. Track Detect. 1978, 1, 189.
- Nouh, S.A.; Abdel Salam, M.H., Ahmed Morsy, A. Radiat. Meas. 2003, 37, 25.
- Kobzev, A.P..; El-Asserb, M.R.; El-Halemb, A.A.; Abdul-Ghapharb, U.S.; Salamab T.A. Radiat. Meas. 2003, 37, 201.
- Chapiro, A. Radiation Chemistry of polymeric Systems. Wiley Interscience, New York, 1962, 15.
- Yamauchi, T. Radiat. Meas. 2003, 36, 73.
- Singh, S.; and Prasher, S. Nucl. Instrum Methods B 2004, 222, 518.
- Chung, J.Y.J. Med. Plast. Biomater. Magazine Tech. Paper series, 1977.
- Malek, M.A. and Chong, C.S., Radiat. Meas. 2002, 35(2), 109.
- Yamauchi, T.; Nakai, H., Somaki, Y.; and Oda, K. Radiat. Meas. 2003, 36, 99.
- Singh, S. and Prasher, S. Nucl. Instrum Methods B 2006, 244, 252.
- Sonkawade, R.G.; Kumar, V.; Kumar, L.; Annapoorni, S.; Vaijapurkar S.G. and Dhaliwal, A.S. Indian J. Pure and Appl. Phy, 2010, 48, 453.
- Tuasikal, M.A.; Alothman, O. Y.; Luqman, M.; Al-Zahrani, S. M.; and Jawaid, M. Inter. J. Polym. Anal. & Character. 2013, 19, 189.

This work is licensed under a Creative Commons Attribution 4.0 International License.









