Synthesis, characterization and In-Vitro cytotoxic studies of 5,10,15,20 tetra pyridyl porphyrin coordinated to four [Ru (bipy)2 Cl]+groups
S. Uthayanila1 , P. Neeraja2
1Department of Chemistry, AVS Engineering College, Salem, Tamil Nadu, India - 636003
2Department of Chemistry, Adhiyamaan College of Engineering, Hosur, Tamil Nadu, India - 635109
DOI : http://dx.doi.org/10.13005/ojc/310229
Article Received on :
Article Accepted on :
Article Published : 06 Jun 2015
Ruthenium possesses several favorable properties suited to rational anticancer drug design when conjugation with the porphyrin moiety was accomplished through peripheral pyridyl rings. The ruthenium porphyrin conjugates are soluble at least moderately in aqueous solution and are thus suitable for biological investigations in particular for cytotoxicity and photocyotoxicity tests. In present study the compound 5,10,15,20 tetra pyridyl porphyrin coordinated to four [Ru (bipy)2 Cl]+ groups (meso-5,10,15,20 tetrakis {4(chloro-bis-bipyridyl ruthenium(II)) pyridyl} porphyrin) is synthesized by modified Alder method. This compound is characterized by UV-Visible Spectroscopy, FT-IR Spectroscopy, 1H-NMR spectroscopy, Fluorescence Spectroscopy and Cyclic Voltametry. In-Vitro anticancer activity of the compounds have been evaluated by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay method. The results show that the compound is cytotoxic against human lymphoma cancer cells.
KEYWORDS:Cytotoxic; FT-IR; Cyclic Voltametry; Fluorescence; UV-Visible; NMR
Download this article as:| Copy the following to cite this article: Uthayanila S, Neeraja P. Synthesis, characterization and In-Vitro cytotoxic studies of 5,10,15,20 tetra pyridyl porphyrin coordinated to four [Ru (bipy)2 Cl]+ groups. Orient J Chem 2015;31(2). |
| Copy the following to cite this URL: Uthayanila S, Neeraja P. Synthesis, characterization and In-Vitro cytotoxic studies of 5,10,15,20 tetra pyridyl porphyrin coordinated to four [Ru (bipy)2 Cl]+ groups. Available from: http://www.orientjchem.org/?p=9080 |
Introduction
Porphyrins and porphyrin like compounds are of special interest because of their many unique properties and technological applications 1-5. The conjugation of the porphyrins to metal fragment is a strategy for making new compounds that are expected to combine the photo toxicity and the tumor localization properties of the porphyrin chromophore with the cytotoxicity of the metal fragment for additive antitumor effect 6.The presence of metal ions and metal coordinated compounds are known to affect cellular process in a dramatic way. This metal effect influences not only natural processes, such as cell division and gene expression, but also non natural processes, such as toxicity, carcinogenicity and antitumor activity 7.
Pyridyl porphyrins are intriguing ligands that yield, when coordinated to metal porphyrin complexes or to other metal complexes, unique supramolecular assemblies with multi electron redox and photochemical activities8 .The transition metal centers are biologically active. It was reported that tetra ruthenated porphyrin can bind to DNA with high affinity .The organometallic fragments are modulate the biological properties of porphyrin complexes. The addition of the organometallic fragments increases the hydrophilicity of the highly hydrophobic porphyrin ligand 9-14
Experimental
Materials
All the chemicals and reagents used were analar grade obtained from Merck, India and were used as received without further purification. RPMI Medium, penicillin and streptomycin antibiotic solutions and fetal boivine serum (FBS) were purchased from ATCC (USA). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), dimethyl sulfoxide (DMSO) were purchased from Invitrogen (Carlsbad, CA, USA) and Sigma-Aldrich (St. Louis, MO, USA).
Synthesis
Synthesis of 5,10,15,20 tetra pyridyl porphyrin (TPyP)
Synthesis of TPyP was done according to literature data15 : A mixture of 9.41 ml (100 mmol) of 4-pyridinecarboxaldehyde and 13 ml (100 mmol) propionic anhydride was dissolved in 300 ml propionic acid and 6.93 ml (100 mmol) pyrrole dissolved in 100 ml of propionic acid, was added drop wise to the mixture heated to reflux, for 1/2 h. The whole reaction mixture was refluxed for over 2h. After cooling to room temperature, the solvent was evaporated to dryness, and the oily residue was repeatedly washed with hot water, neutralized by aqueous ammonia (25%), and washed again with hot water. The purple solids obtained by this procedure were filtered and dried.
Synthesis of Meso-5,10,15,20-tetrakis {4(chloro-bis-bipyridylruthenium(II)) pyridyl} porphyrin [µ-(H2TPyP) { Ru- (bpy)2 Cl}4]4+
Step-I: Synthesis of Cis-Ru(bipy)2Cl2
Synthesis of Cis-Ru(bipy)2Cl2 ,was done according to the literature data 16. A mixture of RuCl3.3H2O, LiCl and 2,2’ bipyridine were refluxed in DMF for 8 hour under an Ar atmosphere. After the reaction mixture was cooled to room temperature, acetone was added and the resulting mixture was left 00C overnight. The dark purple mixture obtained was then filtered, and washed with ether.

Step-II: Meso-5,10,15,20 tetrakis {4(chloro-bis-bipyridyl ruthenium(II)) pyridyl} porphyrin [µ-(H2TPyP) { Ru- (bipy)2 Cl}4]4+
Mixture of Cis-Ru(bipy)2Cl2,(0.32 g, 0.64mmol) and TPyp (0.1g,0.161 mmol) was refluxed for 45 min in glacial acetic acid (10 cm3) under an Ar atmosphere. After removing the solvent in a flash evaporator, the solid was redissolved in a minimum quantity of methanol and refluxed for 45 min. The products were precipitated by adding acetone saturated with LiCl and collected on a filter. A dark red solid was obtained. The crude product was placed on a silica gel column, and eluted with CHCl3: EtOH solution (V/V, 20:1).The second brown band was collected and dried under vacuum. Yield: 34%
 |
Scheme Click here to View scheme |
Anticancer Activity Studies
Cell culture
The U937 (human histiocytic lymphoma cell line) was procured from American Type Culture Collection (ATCC, USA). The cells were cultured in RPMI medium with 10% Fetal bovine serum (FBS), 100 U/mL penicillin and 100 μg/mL streptomycin in T25cm2, T75 cm2, and 96-well culture plates at 37°C in a humidified atmosphere containing 5% CO2. All experiments were performed using cells from passage 15 or less.
Cell viability assay
The MTT assay was carried out as previously described by Mossman (1983). Briefly, U937 cells were plated at a density of 1.5 x 104 cells per well in 200μL of fresh culture medium. After overnight growth, cells were treated at different concentrations (25–125 μg/mL) of complexes 1-3for 24 and 48 h. After incubation, 20 μL of MTT solution (5 mg/mL in phosphate-buffered saline (PBS) were added to each well. The plates were wrapped with aluminum foil and incubated for overnight at 37 °C. The plates were centrifuged and supernatant was carefully removed. Purple color formazan crystals were dissolved by the addition of 100μL of DMSO to each well. The absorbance was monitored at 570 (measurement) and 630 nm (reference) using a 96-well plate reader (Bio-Rad, CA, USA). Data were collected for three replicates each and used to calculate the mean. The percentage inhibition was calculated, from this data, using the formula:

Results and Discussion
UV-Visible Spectroscopy
A small perturbation of pyridyl porphyin spectra after the coordination of four [Ru(bpy)2 cl]+groups. However three new absorption bands to the characteristic spectra (Fig.1) of porphyrin at 250 nm, 287nm and 352 nm assigned to 2,21 bipyridine intraligand pπ-pπ transition and two peak 402 and 503nm due to Ru(dπ)-bpy(pπ) charge transfer transitions respectively.
![Fig. 1 UV-Visible spectrum of [µ-(H2TPyP) { Ru (bpy)2 Cl}4]4+](http://www.orientjchem.org/wp-content/uploads/2015/06/Vol31_No2_Synth_S.Utha_Fig1-150x150.jpg) |
Figure1: UV-Visible spectrum of [µ-(H2TPyP) { Ru (bpy)2 Cl}4]4+ Click here to View figure |
Fourier transforms infrared (FT-IR) Spectroscopy
Absorption peaks (Fig. 2) obtained at~ 1240 cm-1for [µ-(H2TPyP) {Ru- (bpy)2 Cl}4]4+ assigned to N-H stretching and in-plane deformation vibrations respectively.Absorption bands assigned to C=C and C=N skeletal modes of porphyrins located between 1640 cm-1 and 1420 cm-1. The peaks at 796 cm-1 – 780 cm-1 due to C-H out-plane bending vibrations in pyrrole and pyridine rings. The peak appears at 1036 cm-1 dut to C=N stretching vibration of pyridine in [µ-(H2TPyP) {Ru- (bpy)2 Cl}4]4+, this may be due to coordination of ruthenium bipyridine group in pyridine. The peaks appears at 443 cm-1 for [µ-(H2TPyP) {Ru- (bpy)2 Cl}4]4+ due to metallation of Ru.
![Fig. 2 FTIR spectrum of [µ-(H2TPyP) { Ru (bpy)2 Cl}4]4+](http://www.orientjchem.org/wp-content/uploads/2015/06/Vol31_No2_Synth_S.Utha_Fig2-150x150.jpg) |
Figure2: FTIR spectrum of [µ-(H2TPyP) { Ru (bpy)2 Cl}4]4+ Click here to View figure |
NMR studies
The Proton NMR spectra of [µ-(H2TPyP) {Ru- (bpy)2 Cl}4]4+ (Fig. 5) shows doublet at 9.975 ppm corresponds to 8 identical protons at pyrrole ring. Two doublets at 8.64 ppm and 8.48 ppm correspond to pyridyl protons α and β to N – atom respectively. The triplets at 8.07 ppm assigned to bipyridyl protons meta and triplet at 7.77 ppm assigned to bipyridyl protons para to N-atom attached to Ruthenium metal ion. The other peaks between 7.79 ppm to 7.09 ppm assigned to other bipyridyl protons. The diamagnetic ring current shields the N-H protons, therefore it shifts to upfield, even further than the tetramethylsilane (TMS) peak 17
Table 1: Cytotoxic Activity data of [µ-(H2TPyP) { Ru- (bpy)2 Cl}4]4+
| Table 1 – [µ-(H2TPyP){ Ru (bpy)2 Cl}4]4+ | |||
|
S. |
Dose |
% Death |
% Death |
|
1 |
0 |
0 |
0 |
|
2 |
25 |
5.39 |
13.14 |
|
3 |
50 |
8.82 |
20.54 |
|
4 |
75 |
16.84 |
31.18 |
|
5 |
100 |
21.98 |
38.79 |
|
6 |
125 |
38.49 |
52.78 |
Cyclic voltammetry studies
Tetra ruthenated tetra pyridyl porphyrin is cycled in the anodic direction there is a quasi-reversible redox couple with +0.88V associated with Ru (III/II). In the cathodic direction two quasi reversible redox couple with -0.68V and -1.11V are porphyrin centered redox process (Fig. 3). An additional quasi reversible redox couple with -1.42V is present in the cyclic voltagram of the ruthenated porphyrin solution, but it’s not present in the free base porphyrin cyclic voltagram. This redox couple is attributed to the bipyridine ligands coordinated to the Ru(II) centers. The area under the redox wave is associated with the Ru(II) center is approximately twice the area of the first reduction process. This agrees with the two to one ruthenium to porphyrin stoichiometry predicted for complex redox couple lies intermediate to the free base porphyrin and metal complex. Stabilization of the porphyrin lowest occupied molecular orbital (LUMO) by the peripheral Ru groups.
![Fig. 3 Cyclic voltagram [µ-(H2TPyP) { Ru (bpy)2 Cl}4]4+](http://www.orientjchem.org/wp-content/uploads/2015/06/Vol31_No2_Synth_S.Utha_Fig3-150x150.jpg) |
Figure3: Cyclic voltagram [µ-(H2TPyP) { Ru (bpy)2 Cl}4]4+ Click here to View figure |
Fluorescence studies by Spectroflourimetry
The coordination of four [Ru (bipy)2Cl]+ groups to the pyridyl nitrogen atoms decrease the fluorescence quantum yield by two and three orders of magnitude. This should arise from the thermal relaxation and inter system crossing efficiencies induced by the peripherally bound ruthenium complex as well as the higher dipolemoment. In supramolecular porphyrins, this effect is shadowed by the heavy atom effect and tuned by the electronic coupling between the porphyring ring and ruthenium complexes. The emission spectra (Fig. 4) reveals that the complexes were excited at different excitation wavelengths, it was put in to evidence that the intensity of the emission spectra is in accordance with the magnitude of energy of the excitation.
![Fig. 4 Emission spectrum of [µ-(H2TPyP) { Ru (bpy)2 Cl}4]4+](http://www.orientjchem.org/wp-content/uploads/2015/06/Vol31_No2_Synth_S.Utha_Fig4-150x150.jpg) |
Figure4: Emission spectrum of [µ-(H2TPyP) { Ru (bpy)2 Cl}4]4+ Click here to View figure |
![Fig. 5 NMR spectrum of [µ-(H2TPyP) { Ru (bpy)2 Cl}4]4+](http://www.orientjchem.org/wp-content/uploads/2015/06/Vol31_No2_Synth_S.Utha_Fig5-150x150.jpg) |
Figure5: NMR spectrum of [µ-(H2TPyP) { Ru (bpy)2 Cl}4]4+ Click here to View figure |
In- vitro cytotoxic studies
Our ultimate aim was to find an efficacious anticancer drug screening of the complex [µ-(H2TPyP) {Ru- (bpy)2 Cl}4]4+ by appropriate design of the structure of the complex to fulfill the requirements towards pharmacological profiles such as membrane permeability, target delivery, and modulation of cancer cell death. The cytotoxic effects of as prepared complex were examined on cultured U937 human histiocytic lymphoma cell line by exposing cells for 24 hour and 48 hour to a medium that contained the respective complexes at 25, 50, 75, 100, 125 µg/mL for 24 hour and 48 hour. Cell viability was reduced by approximately 40 % to 50 % for [µ-(H2TPyP) {Ru- (bpy)2 Cl}4]4+ concentrations at higher dose 125 µg/mL after treatment for 24 hour and 48 hour. The cytotoxic results of complex [µ-(H2TPyP) {Ru- (bpy)2 Cl}4]4+ at 48 hr treatment has showed that significantly decrease in a concentration-dependent and time dependent manner to approximately 20% compared to the 24 hr treatment (Figure 6)
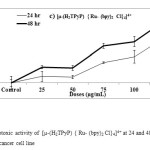 |
Figure6: Cytotoxic activities of material 3 at 24 and 48 hr on U937 lymphoma cancer cell line Click here to View figure |
The complex is cytotoxic to human lymphoma cancer cells. As seen in this preliminary study from MTT assay, it showed desired and valuable starting points for selecting a lead candidate for anticancer drug designing. However, it would be pertinent to test these materials against several more tumor cell types and to undertake in depth studies to dissect the molecular mechanisms of action of these materials that induce apoptosis (target based cancer therapy).
References
- Srishailam.A, Yata Praveen Kumar, Nazar M. D. Reddy.P, Deepika.N, Nageti Veerababu, Satyanarayana.S ,Journal of Fluorescence,2013 23, 897-908
- Yin Xie, Zheng Li, Gan-Jian Lin,Liang Huang, Zhen Wang, Hua Liang, Bin Jiang, Jun Liu, Inorganica Chimica Acta.2013 405, 228-234
- Machado.E.H ,Gomes.R,Diesley ,.M.S.Araujo, Higlio.S,veno.T, Rodrigo De cavalerio.A.S,newton M.Barbosa Neto, Molecules 2011,16,5807-5821
- Bruno Therrien,Top curr.chem,2012,319,35-36
- Irigoyen.J.R, Balaco.L.M, Lopez.S.T, Int.J.Electrochem.sci. 2012, 7, 11246-11256
- Bratsos.I,Lengo.E,Milani.B,Ostric.A,Spagnul.C,zangrando.E,Alessio.E,Dalton Trans,2009,48,10742-10756
- Irena Kostava,Recent patents on Anti-cancer Drug Discovery,2006,1,1-22
- Kalyanasundaram.k,Inorg.Chem,.1984,23,2453-2459
- Mei W-J,Wei X-Y,Liu J,Lu W-G,Transition Metal chemistry,2007,32,685
- Wei La, Bin Jiang, Hua Yao,Zhen Wang, Ji wang,Jie HanYin Xie, Jian Lin, Liang Huang, Jun Liu, Journal of photochemistry and photobiology B:Biology,2014,140,94-104
- Yao Zhang, Andy Ho,Jiping Yue, Linlin Kong, Zuping Zhou, Xiaoyang Wu, Feng Yang, Hong Liang, European Journal of Medicinal chemistry,2014, 86,449-455
- Yanan Liu,Tianfeng chen,Shing Wong,Jie Mei, Mei Huang, Fang Yang, Jie Liu,Jie Zheng, ,Chemico-Biological Interactions, 2010,83, 349-356
- Ashish Kumar Singh,Daya shankar Pandey,Qiang Xu,Pierre Braunstein,Chemistry Reviews, 2013,270-271, 31-56
- Frederic Schmitt, Barry.P.E,Jeanneret, Bruno Therrin, Bioinorganic and Medicinal chemistry Letters,2012, 22, 178-180
- Aviezer.D,Cotton.S,David.M,Segev.A,Khaselev.N,Galili.N,Gross.Z,Yayon.A, Cancer Research, 2000,60,2973,
- Sullivan.B.S,Salmon.D.J and Meyer.J.J,Inorg.Chem,1978,17,3334
- Milgrom, L. R. The Colours of Life: An Introduction to the Chemistry of Porphyrins and Related Compounds; Oxford University Press Inc.: New York, 1997.

This work is licensed under a Creative Commons Attribution 4.0 International License.









