Removal of Cu(II) from Aqueous Solutions using Dried Activated Sludge and Dried Activated Nano-Sludge: Adsorption on a Fixed-Bed Column
Zahra Ahmari1*, Morteza Khosravi2 And Ali Niazi3
1,2 Department of Applied Chemistry , Faculty of Chemistry North Tehran Branch , Islamic Azad University, Tehran , Iran 3Department of Chemistry, Faculty of Science, Arak Branch, Islamic Azad University, Arak, Iran. Corresponding author E-mail: z_ahmari@iau-tnb.ac.ir
DOI : http://dx.doi.org/10.13005/ojc/310238
Article Received on :
Article Accepted on :
Article Published : 24 Jun 2015
In the present time study,dried activated sludge(DAS) and dried activated nano sludge(DANS) was used for the removal of Cu(II) from aqueous solution in a fixed-bed reactor. The effect of important parameters including the flow rate and bed height were examined. Dried activated nano sludge column regeneration using 1 M concentration of HNO3 has been studied.The Thomas model was used for the mathematical description of the adsorption of Copper at different flow rate and various bed height to determine parameters of the column suitable for process design. Both exhaustion time and breakthrough time increased with increasing bed height, while the adsorption bed capacity decreased as the flow rate increased.
KEYWORDS:Dried activated sludge; Copper; Thomas model; Regeneration; Fixed-bed
Download this article as:| Copy the following to cite this article: Ahmari Z, Khosravi M, Niazi A. Removal of Cu(II) from Aqueous Solutions using Dried Activated Sludge and Dried Activated Nano-Sludge: Adsorption on a Fixed-Bed Column. Orient J Chem 2015;31(2). |
| Copy the following to cite this URL: Ahmari Z, Khosravi M, Niazi A. Removal of Cu(II) from Aqueous Solutions using Dried Activated Sludge and Dried Activated Nano-Sludge: Adsorption on a Fixed-Bed Column. Available from: http://www.orientjchem.org/?p=9398 |
Introduction
Heavy metals pollution are present in industrial wastewater and are discharged in the enviroment. The presence of toxic heavy metals in aqueous source is one of the most important problems that effectiveness in to enviroment is very important1,2.Copper is widely used in various industrial activities such as metal plating, mining, tannering and vehicle industry3.
These applications have introduced Cu into aquatic ecosystems, which cause serious environmental pollution problems and bring harmful effect to living organisms. Therefore, it is necessary to reduce Cu (II) from surface waters.
Chemical methods for the removal of heavy metals from wastewater include electrochemical removal, chemical precipitation, ion exchange and memberance. Some of the toxic sludge or biological sludge and can,t be regenerated4,5 .
Today, biosorption of heavy metals from industrial wastewater can be considered as an alternative method, due to high removal efficiency and without yielding harmful by-products6.
Adsorbent materials obtained from suitable can be used effectively in the removal heavy metal ions7.Biological removal of heavy metal ions with high efficiency, high adsorption capacity, easy fixation and fast adsorption-desorption kinetics. Biosorption is a property of certain types of inactive, dead microbial biomass to bind and concentrate heavy metals from very dilute aqueous solutions8.
Activated sludge from wastewater treatment processes is probably the most abundant source of mixed biomass. The ability of activated sludge biomass to remove and accumulate heavy metals and organics has been recognized in different studies9. Excess sludge from such wastewater systems can be separated and utilized for removal of heavy metal ions as an abundant and cheaper biosorbent10. Due to the adsorptive capacity of the microorganisms for heavy metal ions, the biomass can be also be successfully used as a sorbing11,12. Capability of microorganisms to bind heavy metals in aqueous solutions has long been of scientific interest13. Homaidan et al. (2014) studied the removal of Copper ions from aqueous solutions by Spirulina platensis biomass14. Abdel Aty et al(2013) evaluated the potential of removing cadmium and lead from aqueous solution using fresh water alga Anabaena Sphaerica biomas15.
In this study, removal of Copper by dried activated sludge (biomass) and activated nano sludge (nano-biomass) has been studied in fixed bed column reactor. For heavy metal biosorption in packed bed reactors, cells are generally immobilized to improve their structural strength, rigidity and porosity 16. In order to predict the breakthrough curve of an adsorption process in a fixed-bed the Thomas model has been often used17.
Important design parameters such as column bed height and flow rate of metal solution in to the column have been evaluated. The efficiency of removal and recovery Cu(II) as a desorption process from adsorption column was evaluated at different operation condition.
Material and Methods
Preparation of Biosorbent and Copper Solution
Activated sludge was obtained from Shazand Petrological Co. (Arak, Iran). The activated sludge was refluxed for 60 min with HNO3 1 M and sieved through a Watman paper, and it was washed with distilled water. Then, the activated sludge was refluxed for 60 min with NaOH 1 M.
The product was washed with the distilled water and then dried at room temperature (25°C).
The dried biomass was grinding to yield a powder. Activated sludge was characterized with scanning electron microscopy (model EM 3200 made KYKY). Figure 1a, b shows SEM pictures of the adsorbents (DAS and DANS) that particles size distribution of the activated nano sludge in uniform and homogeneous shapes. The experiment solutions were prepared by diluting 1000 mg/L of Cu (NO3)3⋅3H2O standard solution with deionized water to the desired concentrations (10-100mg/L) of Cu(II). All chemicals used in this work were of analytical reagent grade.
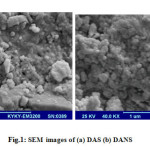 |
Figure1: SEM images of (a) DAS (b) DANS |
Immobilization of Activated Sludge
Activated sludge was immobilized in sodium alginate polymer beads. Immobilization of activated sludge biomass was carried out by the entrapment method. Na-alginate solution of 3% was prepared by dissolving 0.4 g of Na-alginate in 20 mL of hot distilled water with constant stirring to avoid aggregation formation. The mixture was cooled to 400C and activated sludge added in weigh ratio of 1:2 under stirring condition to have a uniform mixture. The slurry was extruded by syringe into 0.2 mol/L calcium chloride solution under shaking by using a peristaltic pump. After 35 min, the bead were amassed from solution and washed with deionized water. Then beads were placed into a solution of calcium chloride (0.2mol/L) for 10 min, through Ca2+ diffused into the beads and produced the gelification on the bead surface. After that, the beads were washed with deionized water and kept in a solution of calcium chloride (0.2 M) for 60 min at 40C and finally washed with NaCl 0.7 %18,19.
Column Set Up
The fixed-bed column were performed in a glass tube with internal diameter of 2/5 cm and length of 50cm.About 20 cm of its height has been packed with immobilized active sludge,
And a valve has been fitted at the bottom of the column. The pH of the solutions was maintained constant at 3.0. Biosorption experiments conducted in a fixed-bed column at 250C and effluent concentration(25mg/L). The Cu(II) concentration at the outlet of the column at various time periods was determined using atomic absorption spectrometer (model AA 680 made of SHIMADZU) at 324/7 nm.
Mathematical Model
Several models illustrate the dynamic behavior of biosorption in a fixed-bed system that based on concentration-time curves20.In this study, the Thomas model has been applied to biosorption columns. The Thomas model is one of the most general and widely applied to demonstrate the operation and predicted of breakthrough curves of the biosorption process in fixed bed column. The linearized form of the Thomas model can be expressed by the following equation:

Where KTh (mL/min mg) is the Thomas model constant, q0(mg/g) is the amount of metal ion concentration adsorbed by biomass, C0(mg/L) is the influent heavy metal ion, Ct(mg/L) is the effluent metal ion concentration, W(g) is the mass of adsorbent in the fixed- bed column and Q(mL/ min) is the flow rate. The parameters KTh and q0 may be determined from a plot of 1n(C0/Ct-1) versus time.
Results and Discussion
Effect of Flow Rate on The Biosorption of Copper
The investigation on the effect of flow rate on the biosorption of Cu(II) on immobilized dried activated sludge and dried activated nano sludge, the influent metal concentration was held constant at 25 mg/L for Cu (II) , bed height (10 cm) constant and changing flow rate 2 to6 mL/min.The breakthrough curve shown in Fig.2 was a plot of dimensionless concentration (Ct/C0) versus time(t). It was shown that breakthrough curve becomes steeper when the flow rate is increased with which the break point time and exhaustion time decreased.
At higher flow rates, metal ions had less time to contact with microorganism that resulted in lower removal efficiency of metal ions in column. While increasing the flow rate, the result shown that the biosorption capacity could reach the equilibrium value faster, which may cause an increase in mass transfer rate and biosorbent biomass acquires saturated in the early hours21.Table 1 show, the parameters Thomas model at the various flow rates.
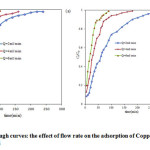 |
Figure2: Breakthrough curves: the effect of flow rate on the adsorption of Copper onto (a) DAS and (b) DANS |
Table1: Column parameters with various flow rate and constant bed height (10cm) for adsorption of Cu (II) onto DAS and DANS
|
R2 |
te (min) |
q0 (mg/g) |
KTh (mL/min.mg) |
Flow rate (mL/min) |
Adsorbent |
|
0.993 |
80 |
2.1 |
3×10-3 |
2 |
DAS
|
|
0.991 |
50 |
1.8 |
6.55×10-3 |
4 |
|
|
0.986 |
35 |
1.2 |
7.05×10-3 |
6 |
|
|
0.963 |
150 |
3.8 |
1.7×10-4 |
2 |
DANS |
|
0.978 |
90 |
3.45 |
2.7×10-3 |
4 |
|
|
0.983 |
50 |
2.8 |
4.2×10-3 |
6 |
Effect of Bed Height on the Biosorption of Copper
The effect of bed height on the biosorption of Cu(II) using various bed heights 10,15 and 20 cm, as shown in Figure 3. Accumulation of Copper(II) ion (25mg/L) in the packed bed column is mainly dependent on the quantity of immobilized biosorbents (biomass alginate) inside the fixed bed column. As bed height increases, the breakthrough point time increases and the amount of copper adsorbed on biosorbent surface also increases. This is mainly due to the higher contact time between metal ion solution and DAS and DANS surface22.
Increasing in bed height from 10 to 20 cm has signification effect on removal efficiency and metal uptake capacity23. Table 2 show, the parameters Thomas model at the various bed heights.
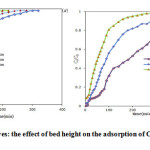 |
Figure3: Breakthrough curves: the effect of bed height on the adsorption of Copper onto (a) DAS and (b) DANS |
Table2: Column parameters with various bed height and constant flow rate (2ml/min) for adsorption of Cu(II) onto DAS and DANS
|
R2 |
te (min) |
q0 (mg/g) |
KTh (mL/min.mg) |
Bed height (cm) |
Adsorbent |
|
0.954 |
130 |
2.9 |
2×10-3 |
15 |
DAS
|
|
0.979 |
150 |
3.4 |
1.85×10-3 |
20 |
|
|
0.977 |
190 |
4.2 |
1.45×10-3 |
15 |
NDAS |
|
0.989 |
220 |
5.5 |
1.2×10-3 |
20 |
Column Regeneration Studies
Regeneration of the adsorbent materials is of crucial importance in the economic success of biosorption technology development24.When the beads containing biomass became saturated with metal ion, the cheapest method of recovering metals from the surface of biomass is to strip
or elute the metal from the surface of biomass by desorption agent such as acid treatment18.
In this study, elution of the Cu(II) from aqueous solution was done using concentration of nitric acid solution 1 M. Desorption curves presented in Fig.4 showed the concentration of metals in the eluting. Cu((II) is easily desorbed because in less than 90 minute the desorption nearly completed. The majority of the adsorbed Cu ions from the column eluted in approximately 65 min. These data show that more than 94% of adsorbed metal can be easily eluted from the packed bed column. The acid wash had no visible effects on the physical properties of the beads.
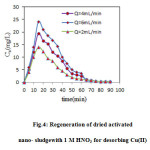 |
Figure4: Regeneration of dried activated nano- sludgewith 1 M HNO3 for desorbing Cu(II) Click here to View figure |
Conclusions
This study showed that the dried activated sludge and dried activated nano sludge could be used as an efficient biosorbent for the removal of Copper ions from aqueous solutions. The experimental data obtained from the study showed that DANS is more effective adsorbent than DAS to removal of Copper ion from aqueous solution. The removal efficiency of Cu(II) ions from aqueous solutions strongly dependent on the flow rate and bed height. With increasing flow rate the removal efficiency of Cu (II) decreased, while an increase in bed height enhanced the removal efficiency. The Thomas mathematical model was used for the column data obtained from the adsorption of Cu (II) ions onto DAS and DANS. The adsorbed Cu(II) ion be effectively eluted with the use of concentration of nitric acid solution 1 M.
Refrences
- Kapoor, A.; Viraraghavan, T.; Cullimore, D. C. Bioresourc Technology . 1999, 70, 95– 104.
- Manjula Devi, M.; S, Manonamni. Oriental journal of Chemistry. 2015, 31, 531-539.
- Khan, S.; Cao, Q.; Zheng, Y. M.; Huang, Y.Z.; Zhu, Y.G. China environment Pollution. 2008, 52, 686-692.
- Sud, G. M.; Kumar, M. P. Bioresource Technology. 2008, 99, 6017-6027.
- Chaouch, N.; Chaouch, M.; Ouahrani ,R.; Laouini, S. E. Oriental journal of Chemistry.2014, 30, 1317-1322.
- Eren, E. Journal of Hazardous Material. 2008,159, 235-244.
- Yan, G.; Viraraghavan, T. Water Research. 2003, 37, 4486–4496.
- Aksus, Z.; Gulen, H. Process Biochemistry. 2002, 38, 161-173.
- Al Asheh, S.; Duvnjak, Z. Biotechnology.1995, 91, 83-87.
- Kadirvelu, K.; Thamaraiselvi, K.; Namasivayam, C. Bioresource Technology. 2001, 76,63-65.
- Tsczos, M.; Bell, J .P. Water Research .1989, 23, 563-568.
- Brandt, S.; Zeng, A.; Deckwer, W. Biochemical Engineering and Biotechnology. 1997, 55, 480-489.
- Mahmoud, M. E.; Yakout, A. A.; Abdel-Aal, H.; Osman, M. M. Desalination. 2011,279, 291–297.
- Al Homaidan ,A. A .; Al Houri, H. J.; Al Hazzani, A. A.; Elgaaly, G.; Moubayed , N . M. S. Arabian Journal of Chemistry .2014, 7, 57-62.
- Abdel Aty, A. M.; Ammar, N. S.; Ghafar, H. H. A.; Ali, R. K. Journal of Advanced Research. 2013,4, 367-374.
- Lee, S.H.; Jung, C.H.; Chung, H. Process Biochemistry.1198, 33,205-211.
- Yan, G.; Viraraghavan, T. Bioresource Technology.2001,78,243-249.
- Marandi, R. Canadian Journal on Chemical Engineering and Technology. 2011,2, 8–22.
- Wu, H.S.; Zhang, A.; Wang, L.S. Journal Enviromental Science .2004, 4, 640-645.
- Park, D.; Yun, Y. S.; Park, J. M. Enviromental Science Technology. 2004, 38, 4860-4864.
- Han, R.; Zhang, J.; Zou, W.; Xiao, H.; Shi, J.; Liu, H. Journal of Hazardous Materials. 2006, B133, 262–268.
- Preetha, B.; Viruthagiri, T. Seperation and Purification Technology.2007, 57,126-133.
- Sivaprakash, B.; Rajmohan, N.; Sadhik, A. M. Journal of chemtech. Research.2010,12,155-162.
- Goel, J.; Kadirvelu, K.; Rajagopal,C.;Kumar Garg, V. Journal of Hazardous Material. 2005, B125,211-220.

This work is licensed under a Creative Commons Attribution 4.0 International License.









