Photooxidative Removal of Phenazopyridine by UV/H2O2 Process in a Batch Re-circulated Annular Photoreactor: Influence of Operational Parameters
Soudabeh Saeid and Mohammad A. Behnajady*
Department of Chemistry, Tabriz Branch, Islamic Azad University, Tabriz, Iran
DOI : http://dx.doi.org/10.13005/ojc/310279
Article Received on :
Article Accepted on :
Article Published : 30 Apr 2015
In this work, efficiency of UV/H2O2 process in the removal of Phenazopyridine (PhP) as a model contaminant from pharmaceutical compounds was investigated. For this purpose, a suitable batch re-circulated annular photoreactor with a replaceable UV-C lamp in the center of photoreactor was used. Effect of operational parameters such as initial PhP concentration, H2O2 concentration, UV-light source power and pH of the solution was investigated. Results indicate UV/H2O2 process is a powerful method for removal of PhP from aqueous solutions so that a considerable removal of this contaminant has been obtained in the short irradiation time.
KEYWORDS:Advanced Oxidation Processes (AOPs); UV/H2O2; Phenazopyridine; Batch re-circulated annular photoreactor
Download this article as:| Copy the following to cite this article: Saeid S, Behnajady M. A. Photooxidative Removal of Phenazopyridine by UV/H2O2 Process in a Batch Re-circulated Annular Photoreactor: Influence of Operational Parameters. Orient J Chem 2015;31(2) |
| Copy the following to cite this URL: Saeid S, Behnajady M. A. Photooxidative Removal of Phenazopyridine by UV/H2O2 Process in a Batch Re-circulated Annular Photoreactor: Influence of Operational Parameters. Orient J Chem 2015;31(2). Available from: http://www.orientjchem.org/?p=8702 |
Introduction
Development of ecologically clean and safe chemical technologies, processes and materials is vital for maintaining and controlling the environmental pollution1. Such techniques are getting significant attention in academic and industrial researches due to the shortage in clean and natural resources. Beside many other polluters of natural water resources, waste pharmaceutical compounds as a result of conventional disposal of these chemicals through sewer or incineration are very serious polluters2,3. Because of extreme diversity of such chemicals, their elimination and disposal is a very important and challenging effort. Two main approaches undertaken to tackle with this problem include: applying suitable treatments before disposal of waste drugs in to the sewage system and adoption of refinement techniques for elimination of these compounds. However, despite the widespread utilization of these techniques, they do not help the complete removal of drugs from polluted waters. In order to fully eliminate such compounds and purify the contaminated water, new approaches needed to be invented4,5. Advanced oxidation processes (AOPs) are introduced as destructive techniques for water treatment by removal of organic contaminants through oxidative degradation procedures. In general, AOPs involve oxidation of chemicals through ultraviolet radiation in the presence of H2O2, O3, Fenton, or TiO2 which results in an efficient production of hydroxyl radicals6–8. These radicals are highly reactive oxidant with a short life cycle and non-selectively attack to contaminants8–10. Application of hydrogen peroxide in AOPs seems to be more promising among other approaches due to some of its advantages over others such as: it’s complete miscibility with water as well as it’s commercially availability. Moreover, the process can be done in ambient conditions and a whole mineralization of organic carbon into CO2 may take place. In this process, hydroxyl radicals are generated by photolysis of H2O2 through direct absorption of UV light by H2O2 in a photoreactor. The maximum hydroxyl radical yields are obtained by using short-wave ultraviolet radiations (200–280 nm)11.
In this work, the efficiency of UV/H2O2 process in the removal of a Phenazopyridine (PhP) as a model contaminant from pharmaceutical compounds was investigated in a batch re-circulated annular photoreactor. The effect of operational parameters such as initial concentration of PhP, concentration of H2O2, power of light source and pH were studied.
Materials and Methods
Materials
Hydrogen peroxide (37%, w/w) was purchased from Merck (Germany). Phenazopyridine (PhP) with structural formula shown in Fig. 1, is a commonly used drug, and is chosen here as a model drug contaminant. PhP was prepared from Tehran pharmaceutical company (Iran). The presence of the azo group makes PhP as a refractory pollutant12,13.
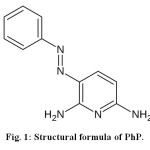 |
Figure1: Structural formula of Ph
|
Photoreactor and Procedure
All experiments were performed in a batch re-circulated annular photoreactor (Fig. 2). The radiation source at annular photoreactor was UV-C lamp (4, 6 and 13 W, λmax= 254 nm, Philips, Holland). In each experiment 500 mL of the PhP and H2O2 solution was transferred to the photoreactor. Then the lamp was switched on to initiate the reaction. During irradiation, the solution was agitated in a constant rate and was transferred with peristaltic pump through annular photoreactor with volumetric flow rate of 140 mL min-1. At certain reaction intervals, 5 mL of sample was withdrawn and the concentration of PhP was determined by means of a spectrophotometer (Shimadzu UV mini-1240).
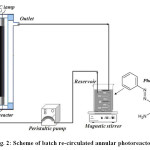 |
Figure2: Scheme of batch re-circulated annular photoreactor. Click here to View figure |
Results and Discussion
Effect of the initial Concentration of PhP
The effect of initial PhP concentration ranging between 20 – 50 mg L−1 on the removal of PhP was investigated. Figure 3 illustrates the result of the experiment with different initial PhP concentrations and fixed amount of hydrogen peroxide. Based on these results, efficiency of PhP removal decreases with increasing the initial PhP concentration. This may be due to the fact that in the higher PhP concentration a stronger inner filter effect makes the solution more impermeable to UV radiation. Hence, efficiency of photooxidative removal of PhP decreases [14].
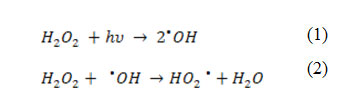
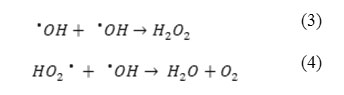
![Fig. 3. Effect of initial concentration of PhP on the photooxidative removal of PhP. P = 6 W, pH = natural, [H2O2] = 500 mg L-1](http://www.orientjchem.org/wp-content/uploads/2015/04/Vol31_No2_Phot_SOUD_Fig3-150x150.jpg) |
Figure3: Effect of initial concentration of PhP on the photooxidative removal of PhP. P = 6 W, pH = natural, [H2O2] = 500 mg L-1 Click here to View figure |
Effect of the Initial Concentration of H2O2
Figure 4 illustrates the effect of initial concentration of H2O2 ranging between 50 – 600 mg L−1 on the removal of PhP. In general, increasing the initial H2O2 concentration increases the efficiency of the UV/H2O2 process. A significant increase in the removal is observed in the range of 50 – 500 mg L−1 from H2O2 and further increasing of the initial H2O2 concentration shows a decrease in the PhP removal percent. In the range of 50 – 500 mg L−1 more available hydroxyl radicals (•OH) in the solution justifies a dramatic increase in the PhP removal percent (Eq. 1). Whereas, further increasing of the H2O2 concentration, lead to produce of HO2• as a result of H2O2 reaction with •OH (Eq. 2) which are less reactive than •OH. Also •OH reacts with HO2• or dimerize to H2O2 (Eqs. 3, 4) that has negative effects on the PhP removal efficiency 14,15.
![Fig.4. Effect of the initial concentration of H2O2 on the photooxidative removal of PhP. [PhP] = 20 mg L-1, P = 6 W, pH = natural](http://www.orientjchem.org/wp-content/uploads/2015/04/Vol31_No2_Phot_SOUD_Fig4-150x150.jpg) |
Figure4: Effect of the initial concentration of H2O2 on the photooxidative removal of PhP. [PhP] = 20 mg L-1, P = 6 W, pH = natural Click here to View figure |
Effect of Light Source Power
As shown in Fig. 5 the removal percent of PhP increases with increasing power of light source from 4 to 12 W. This is justified with higher production rate of •OH at higher UV-light power and thus, an increase in reaction efficiency. According to Eq. (1) higher •OH produces when stronger light source uses in the photoreactor.
Effect of pH
The effect of pH ranging from 3–9.5 on the photooxidative removal of PhP was investigated as shown in Fig. 6. It was observed that the efficiency of the UV/ H2O2 process in the removal of PhP was lower in the pH = 9.5. This can be due to dissociation of H2O2 to the HO2– and H+ at pH = 9.5 according to the Eq. 5. Hence, a decline in the process is rational in the alkaline pH 16.
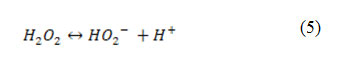
![Fig. 5. Effect of UV light source power on the photooxidative removal of PhP. [PhP] = 20 mg L-1, [H2O2] = 500 mg L-1, pH= natural](http://www.orientjchem.org/wp-content/uploads/2015/04/Vol31_No2_Phot_SOUD_Fig5-150x150.jpg) |
Figure5: Effect of UV light source power on the photooxidative removal of PhP. [PhP] = 20 mg L-1, [H2O2] = 500 mg L-1, pH= natural
|
![Fig. 6. Effect of pH on the photooxidative removal of PhP. [PhP] = 20 mg L-1, P = 6 W, [H2O2] = 500 mg L-1](http://www.orientjchem.org/wp-content/uploads/2015/04/Vol31_No2_Phot_SOUD_Fig6-150x150.jpg) |
Figure6: Effect of pH on the photooxidative removal of PhP. [PhP] = 20 mg L-1, P = 6 W, [H2O2] = 500 mg L-1 Click here to View figure |
Conclustions
In this work, efficiency of the UV/ H2O2 process using a batch re-circulated photoreactor has been studied in the removal of PhP from aqueous solution. The effects of operational parameters such as, initial PhP concentration, H2O2 concentration, UV-light source power and pH have been investigated on the efficiency of the process. According to the results, H2O2 concentration has a critical effect in the removal of PhP and highest removal percent can be obtained by 500 mg L−1 from H2O2 concentration. Also, efficiency of the process in the removal of PhP is higher in the range of 3 – 8 for pH. UV-light source power has also positive effect on the efficiency of reactor and stronger light source causes higher removal percent.
References
- Anpo, M.; Dohshi, S.; Kitano. M.; Hu. Y.; Takeuchi, M.; Matsuoka, M. Annu. Rev. Mater. Res. 2005, 35, 1-27
- Gray, R.; Hogerzeil, H.; Prüs, A.; and Rushbrook, P., Guidelines for Safe Disposal of Unwanted Pharmaceuticals in and after Emergencies, World Health Organization, (1999)
- Emerging Contaminants Workgroup of the Santa Clara Watershed Management Initiative. White Paper: Discussion paper on Pharmaceutical Disposal to Sewer Systems, (2005)
- Pérez-Estrada, L. A.; Maldonado M.I.; Gernjak, W. Catal. Today. 2005, 101, 219-226
- Boreen, A.L.; Arnold, W.A.; McNeill, K. Aquat. Sci. 2003, 65, 320-341
- Colonna, G.M.; Caronna, T.; Marcandalli, B. Dye. Pigment. 1999, 41, 211-220
- Neamtu, M.; Siminiceanu, I.; Yediler, A.; Kettrup, A. Dye. Pigment. 2002, 53, 93-99
- Behnajady, M. A.; Modirshahla, N.; Shokri, M. Chemosphere. 2004, 55, 129-134
- Legrini, O.; Oliveros, E.; Braun, A. Chem. Rev. 1993, 93, 671–698
- Daneshvar, N.; Rabbani, M.; Modirshahla, N.; Behnajady, M. A. Chemosphere. 2004, 56, 895-900
- Lopez, A.; Bozzi, A.; Mascolo, G.; Kiwi, J. J. Photochem. Photobiol. A. Chem. 2003, 156, 121-126
- Herrmann, J.M.; Duchamp, C.; Karkmaz, M.; Hoai, T.B.; Lachheb, H.; Puzenat, E.; Guillard, C. J. Hazard. Mater. 2007, 146, 624-629
- Herrmann, J. Catal. Today. 1999, 53, 115-129
- Basturk, E.; Karatas, M. J. Photochem. Photobiol. 2015, 299, 67-72
- Daneshvar, N.; Rabbani, M.; Modirshahla, N.; Behnajady, M. A. J. Hazard. Mater. 2005, 118, 155-160
- Liu, Y. X.; Zhang, J. Ind. Eng. Chem. Res. 2011, 50, 3836–3841

This work is licensed under a Creative Commons Attribution 4.0 International License.









