NaBH4/Na2C2O4/H2O: An efficient System for Selective Reduction of Aldehydes in the presence of Ketones
Maghsoud Azimzadeh* and Davood Setamdideh
Department of Chemistry, Faculty of Sciences, Mahabad Branch, Islamic Azad University, Mahabad. Iran. E-mail: davood.setamdideh@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/310259
Article Received on :
Article Accepted on :
Article Published : 11 Jun 2015
Selective reduction of a variety of aldehydes (1 equivalents) in the presence of ketones to their corresponding alcohols has been carried out by NaBH4 (1.5 equivalents) & Na2C2O4 (3 equivalents) in water as green solvent in high to excellent yields of the products. An oxalate-borane complex Na2[(H3B)2C2O4] is possibly the active reductant in the reaction mixture. Also, Chemoselective, regioselectivity and exclusive 1,2-reduction enals to their corresponding allylic alcohols in high to excellent yields was achieved successfully with this reducing system.
KEYWORDS:Sodium Oxalate; NaBH4; Selective Reduction; Aldehydes
Download this article as:| Copy the following to cite this article: Azimzadeh M, Setamdideh D. NaBH4/Na2C2O4/H2O: An efficient System for Selective Reduction of Aldehydes in the presence of Ketones. Orient J Chem 2015;31(2). |
| Copy the following to cite this URL: Azimzadeh M, Setamdideh D. NaBH4/Na2C2O4/H2O: An efficient System for Selective Reduction of Aldehydes in the presence of Ketones. Available from: http://www.orientjchem.org/?p=9203 |
Introduction
The selective reduction of aldehydes in the present of ketones is a well-honed strategy in organic synthesis. For this subject numerous reducing reagents and reducing systems have been prepared 1-4 such as a) perform reduction reactions in low temperatures 5-6 b) in the presence of thiols 7 c) by addition of metal salts 8 c) by resins 9 d) on polyethylene glycol 10 e) also by some modified borohydrides 11-13. But, in terms of unavailability of reagents, non-convenient reduction reaction conditions, unacceptable ratio of selectivity and low yields of products, challenges is still remain to investigate of new reducing systems. So, we decided to investigate reducing properties of NaBH4 in the presence of sodium oxalate (Na2C2O4) as a co-reagent for the reduction of aldehydes vs. ketones to their corresponding alcohols. Herein, we wish to report a convenient and efficient system for the reduction of aldehydes vs. ketones to their corresponding alcohols with NaBH4/Na2C2O4/H2Oas a reducing system.
Results and Disscution
Aldehydes are more reactive than ketones in reduction reactions. But it is not sufficient for sole selective reduction of aldehydes vs. ketones as shown in scheme 1, Path A. One approach is to this achievement that the reduction reactions can be carried out slower. For this purpose we have performed the reduction reaction of benzaldehyde as model compound with NaBH4 in the presence of Na2C2O4 in different conditions as shown in table 1. The experiments showed that the reduction reaction of 1 eq. benzaldehyde completed at room temperature in the presence of 3 eq. Na2C2O4 and 1.5 eq. NaBH4 in water as shown in table 1, entry 6.
Table1: The optimization reaction condition for the reduction of benzaldehyde (1 mmol) to benzyl alcohol in water (3 mL) at room temperature with NaBH4 and Na2C2O4.
| Table 1. The optimization reaction condition for the reduction of benzaldehyde (1 mmol) to benzyl alcohol in water (3 mL) at room temperature with NaBH4 and Na2C2O4. | ||||
|
Entry |
NaBH4 (mmol) |
Na2C2O4 (mmol) |
Time (min) |
Conversion (%)a |
|
1 |
1 |
0 |
60-90 |
100<-100 |
|
2 |
1 |
1 |
90 |
100< |
|
3 |
1 |
2 |
90 |
100< |
|
4 |
1 |
3 |
90 |
100< |
|
5 |
1.25 |
3 |
90 |
100< |
|
6 |
1.5 |
3 |
90 |
100 |
|
7 |
2 |
3 |
80 |
100 |
|
a Conversion monitored by TLC and refer to isolated pure product. |
||||
The optimized conditions for reduction of benzaldehye has been used for investigate of selectively reduction reaction. Therefore a mixture of benzaldehyde (1 eq.) and acetophenone (1 eq.) was prepared. Then, the reaction mixture was treated with NaBH4 (1 eq.) and Na2C2O4 (3 eq.) in water (3 mL) at room temperature. After 90 min, we have observed that the reduction of benzaldehye to benzyl alcohols was completed but acetophene was intact material i.e. the competition for reduction was favor of benzaldehyde (scheme 1, path B). This result can not achieve by only using NaBH4 (scheme 1, path A).
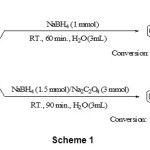 |
Scheme 1 |
The efficiency of this protocol was examined by the reduction of a variety of aldehydes in the presence of different ketones. All reductions were completed within 90-180 min as shown in table 2. The molar ratio of NaBH4 is not different according to the nature of the substrates. 1.5 molar equivalents of NaBH4 and 3 molar equivalents of Na2C2O4 per one equivalents of the substrate were sufficient to complete conversion of aldehydes to the corresponding alcohols in excellent yields (93-95%).
The reduction of conjugated carbonyl compounds is conventional method for the preparation of ally alcohols. However, reduction of α,β-unsaturated aldehydes to their corresponding primary ally alcohols is often difficult due to competing 1,2- vs. 1,4-reduction by the hydride attack. The tendency of sodium borohydride to reduce enals in a conjugate sense is highly dependent on solvent and often ignored.14 However, several specific reagents are available.15 In this context and our continues to development reducing systems, 16 we also investigated the possibility of the 1,2-reduction of α,β-unsaturated aldehydes in the presence of α,β-unsaturated ketones with NaBH4/Na2C2O4/H2O system. The reduction of cinnamaldehyde (scheme 2) and citral (scheme 3) in the presence of benzylideneacetone and chalcone by 1.5 molar equivalents of NaBH4 and 3 molar equivalents of Na2C2O4 were thus carried out exclusively in 1,2-reduction manner within 80-90 minutes at room temperature in water. In this reaction, cinnamyl alcohol and geraniol were obtained. (Table 2, entry 9-12). Cinnamaldehyde and citral showed the best efficiency and regioselective and chemoselective reduction under this protocol.
Table2: Competitive reduction of Aldehydes (1 mmol) vs. Ketones (1 mmol) to their corresponding alcohols by NaBH4 (1.5 eq.)/ Na2C2O4 (3 mmol) in Water (3 mL) at Room Temperature.
|
Table 2. Competitive reduction of Aldehydes (1 mmol) vs. Ketones (1 mmol) to their corresponding alcohols by NaBH4 (1.5 eq.)/ Na2C2O4 (3 mmol) in Water (3 mL) at Room Temperature. |
|||||
|
Entry |
Substrate 1 |
Substrate 1 |
Time/min. |
Conv. 1/ Conv. 2/%a |
|
|
1 |
benzaldehyde |
acetophenone |
90 |
100:0 |
|
|
2 |
4-bromobenzaldehyde |
acetophenone |
90 |
100:0 |
|
|
3 |
4-methylbenzaldehyde |
benzophenone |
100 |
100:0 |
|
|
4 |
4-nitrobenzaldehyde |
acetophenone |
90 |
100:0 |
|
|
5 |
2-methoxylbenzaldehyde |
cyclohexanone |
180 |
100:0 |
|
|
6 |
benzaldehyde |
cyclohexanone |
180 |
100:0 |
|
|
7 |
butanal |
cyclohexanone |
100 |
100:0 |
|
|
8 |
2-hydroxybenzaldehyde |
aceyophenone |
90 |
100:0 |
|
|
9 |
cinnamaldehyde |
benzylideneacetone |
90 |
100:0 |
|
|
10 |
cinnamaldehyde |
chalcone |
90 |
100:0 |
|
|
11 |
citral |
benzylideneacetone |
80 |
100:0 |
|
|
12 |
citral |
chalcone |
80 |
100:0 |
|
.a Conversions refer to TLC monitoring and isolated pure products
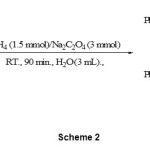 |
Scheme 2 |
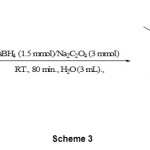 |
Scheme 3 |
The mechanism for the influence of Na2C2O4 is not clear, but as shown in scheme 4 we think that a possible derivative of oxalate-borane as active reductant may form in situ under reaction condition. The possibility to form borane complex via nucleophilic attack is also well-known 17. The oxalate-borane specie is generated by the nucleophilic attack of oxalate ion on borane (scheme 4, II) formed from sodium borohydride and water (scheme 4, I). The oxalate-borate is less reactive than NaBH4 and diminishes reactivity.
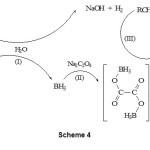 |
Scheme 4 |
All substrates and reagents were purchased from commercially sources with the best quality. IR and 1H NMR spectra were recorded on Perkin Elmer FT-IR RXI and 300 MHz Bruker spectrometers, respectively. The products were characterized by their 1H NMR or IR spectra and comparison with authentic samples (melting or boiling points). Organic layers were dried over anhydrous sodium sulfate. All yields referred to isolated pure products. The purity of products was determinate by 1H NMR. Also, reactions are monitoring over silica gel 60 F254 aluminum sheet.
A typical Procedure
In a round-bottomed flask (10 mL) equipped with a magnetic stirrer, a solution of NaBH4 (0.057 g, 1.5 mmol) and Na2C2O4 (0.402 g, 3 mmol) in water (3 mL) was treated with benzaldehyde (0.106 g, l mmol) and acetophenone (0.12 g, 1 mmol) in one portion. The mixture was stirred at room temperature for 90 minutes. Completion of the reaction was monitored by TLC (Hexane/EtOAc: 9/1). Then, water (5 mL) was added to the reaction mixture. The mixture was extracted with ether (3×10 mL) and dried over anhydrous Na2SO4. Evaporation of the solvent and short-column chromatography of the resulting crude materials over silica gel (eluent; Hexane/EtOAc: 9/1), affords the pure liquid benzyl alcohol as a sole product of reduction reaction and acetophenone as an intact material.
Conclusion
In conclusion, we have shown that NaBH4/Na2C2O4/H2O system reduces a variety of aldehydes vs. ketones to their corresponding primary alcohols in high to excellent yields at room temperature. Reduction reactions were carried out with 1.5 molar equivalents of NaBH4 in the presence of 3 molar equivalents of Na2C2O4 in water as green solvent. All reactions were accomplished with high efficiency of the reductions, using the appropriate molar ratios of NaBH4 andNa2C2O4, convenient reaction times (80-180 min) and easy work-up procedure. Therefore this new protocol for chemoselective & regioselective reduction of aldehydes could be a useful addition to the present methodologies.
Acknowlegment
The authors gratefully appreciated the financial support of this work by the research council of Islamic Azad University branch of Mahabad.
Refrences
- Cha. J. S.; Kim, E. J.; Kwon, O. O.; Kim, J. M. Bull. Korean Chem. Soc., 1996, 17, 50-55.
- Nutaitis, C. F.; Gribble, G. W., Tetrahedron Lett. 1983, 24, 4287-4290.
- Ranu, B. C.; Chakraborty, R., Tetrahedron Lett. 1990, 31, 7663-7664.
- Yumino, S.; Hashimoto, T.; Tahara, A.; Nagashima. H. Chemistry Letters, 2014, doi: 10.1246/cl.140731.
- Ward, D. E.; Rhee, C. K. Synth. Commun. 1988, 18, 1927–1933.
- Ward, D. E.; Rhee, C. K. Can. J. Chem.1989, 67, 1206–1211.
- Maki, Y.; Kikuchi, K.; Sugiyama, H.; Seto, S. Tetrahedron Lett. 1977, 18, 263–264
- Adams, C. Synth. Commun. 1984, 14, 1349–1353.
- Zeynizadeh, B.; Shirini, F. J. Chem. Res., Synop. 2003, 335–339.
- Tanemura, K.; Suzuki, T.; Nishida, Y.; Satsumabayashi, K.; Horaguchi, T. Synth. Commun. 2005, 35, 867–872;
- Kuroiwa, Y.; Matsumura, S.; Toshima, K. Synlett, 2008, 19, 2523–2525.
- Gribble, G. W.; Ferguson, D. C. Chem. Commun. 1975, 535–536.
- Nutaitis, C. F.; Gribble, G. W. Tetrahedron Lett. 1983, 24, 4287–4290.
- Firouzabadi, H; Afsharifar, G. R. Bull. Chem. Soc. Jpn. 1995, 68, 2595-2602.
- (a) Firouzabadi, H.; Tamami, B.;. Goudarzian, N. Synth. Commun. 1991, 21, 2275-2285. (b) Yoon, N. M. ; Choi, J. Synlett. 1993, 135-136. (c) Sim, T. B.; Ahn, J. H.; Yoon, N. M. Synthesis 1996, 324-326.. (d) Bram, G.; Incan, E. D.; Loupy, A. J. Chem. Soc. Chem. Commun. 1981, 1066-1067.
- a) Setamdideh, D.; Rafigh, M. E-J. Chem. 2012, 4, 2338-2345. b) Setamdideh, D.; Rahmatollahzadeh, M. J. Mex. Chem. Soc. 2012, 56, 169-175. c) Setamdideh, D.; Ghahremani, S. S. Afr. J. Chem. 2012, 65, 91-97. d) Setamdideh, D.; Khezri, B.; Rahmatollahzadeh, M. J. Serb. Chem. Soc. 2013, 78, 1-13 e) Mohamadi, M.; Setamdideh, D.; Khezri, B. Org. Chem. Inter. 2013, 2, doi:10.1155/2013/127585. f) Setamdideh, D.; Khaledi, L. S. Afr. J. Chem. 2013, 66, 150-157.g) Setamdideh, D.; Karimi, Z.; Alipouramjad, A. J. Chin. Chem. Soc. 2013, 60, 590-596. h) Setamdideh, D.; Khezri, B.; Esmaeilzadeh, S. J. Chin. Chem. Soc. 2012, 59, 1119-1124.
- a) House, H. O. Modern Synthetic Reactions, 2nd ed.; W. A. Benjamin.: Menlo Park, CA, 1972; pp. 45–54.b) Chandrasekhar, S.; Shrinidhi, A. Synth. Commun. 2014, 44, 2051-2056. c) Ghaderi, S.; Setamdideh, D. Orient. J. Chem. 2014, 30, 1913-1917.

This work is licensed under a Creative Commons Attribution 4.0 International License.









