Facile synthesis of hydroxyapatite particles from cockle shells(Anadaragranosa)by hydrothermal method
Yelmida Azis1, Novesar Jamarun2,*, Syukri Arief2, Hadi Nur3,4
1Department of Chemical Engineering, Faculty of Engineering University of Riau, Pekanbaru, Indonesia.
2Department of Chemistry, Faculty of Mathematics and Science, Andalas University, Padang, Indonesia.
3IbnuSina Institute for Fundamental Science Studies, UniversitiTeknologi Malaysia, 81310 UTM Skudai, Johor, Malaysia.
4Department of Physics, Institut SainsdanTeknologiNasional, Jl. Moh. Kahfi II, Jagakarsa, Jakarta Selatan 12640, Indonesia.
DOI : http://dx.doi.org/10.13005/ojc/310261
Article Received on :
Article Accepted on :
Article Published : 06 Jun 2015
Hydroxyapatite particles, Ca10(PO4)6(OH)2, (HAp), have been successfully synthesized by hydrothermal method using cockle shells (Anadaragranosa)waste as the starting material. The cockle shells were calcined, hydrated (slaking) and undergone carbonation to form precipitated calcium carbonate (PCC).The PCC was added with (NH4)2HPO4 to form HAp by varying the temperatures and reaction times under basic condition (pH 10 – 11). The X-ray Diffraction (XRD)patterns revealed that the excellent product of HAp with hexagonal crystal structure can obtained via facile hydrothermal procedure (140 oC for 16 h). Fourier transform infrared spectroscopy (FTIR) spectra analyses showed the presence of OH, HPO42‒, and PO43‒ absorption bands, indicating the formation of HAp. The dried HAp particles powder was extremely pure with a specific surface area of 17.8 m²/g.
KEYWORDS:cockle shell; hydrothermal; hydroxyapatite; precipitated calcium carbonate
Download this article as:| Copy the following to cite this article: Azis Y, Jamarun N, Arief S, Nur H. Facile synthesis of hydroxyapatite particles from cockle shells(Anadaragranosa)by hydrothermal method. Orient J Chem 2015;31(2). |
| Copy the following to cite this URL: Azis Y, Jamarun N, Arief S, Nur H. Facile synthesis of hydroxyapatite particles from cockle shells(Anadaragranosa)by hydrothermal method. Available from: http://www.orientjchem.org/?p=9095 |
Introduction
Calcium is one of abundant elements in nature both from natural sources (egg shells, corals, cockle shells, bones) and in the form of natural product (limestone) [1,2]. The cockle shell (Anadaragranosa), a lenient animal (mollusca) of bivalvia class (Fig. 1), is one of the potential calcium sources. Moreover, 95% main composition of the shells is in the form of CaCO3 [2]. The cockle shells’ calcium could be processed into the products that are more efficient in the industry or medical field, like Precipitated Calcium Carbonated (PCC) and hydroxyapatite (HAp).
PCC is a calcium carbonate compound (CaCO3) that can be processed from the shells material through a series of chemical reactions. PCC particles’ is homogeneous that in the same size with micro-scale particle [3]. Currently, PCC widely used in industrial project as an addictive of pharmaceuticals, in personal care and cosmetics, for food and beverages, nutritional supplements, paper, plastic, adhesives and sealants, ink, and for some other industries [1,2]. Previously, PCC has been used as a base material in the synthesis of carbonate apatite. Its high level’s purity as well as the small size and constancy of the particles, turn out to be PCC advantage to be changed into apatite compounds [4].
The HAp has been broadly used in the biomedical field for filler and coating of bone and dental implants [5,6]. For this purpose, the HAp should be in very high purity. Besides, because of its spongy structure that possess chemical and thermal resistance, the HAp has also been widely used as a catalyst or adsorbent [7,8,9]. HAp powders can be synthesized via numerous production routes, using a range of different reactants. Some processing techniques include precipitation, hydrothermal, hydrolysis, mechano-chemical and sol–gel process [10-14]. Of these methods the precipitation is the most commonly used synthesis techniques for HAp. However the obtained HAp using this technique, generally, still contain considerable contaminant. The hydrothermal techniques are the second most popular synthesis techniques due to of its simplicity and low-cost. The resulting commonly well crystallized and chemically homogeneous [15]. It is estimated the the high purity of HAp may be produced using PCC as raw material due to the small of it’s particle size. In our previous work, we have modified the process to synthesize the PCC easily through the modified carbonation process [1]. In this study, we synthesized and investigated the properties of HAp from the cockle shells’ PCC using the combination of modified carbonation and hydrothermal methods. To the best our knowledge, the synthesis HAp from PCC, even more cockle shells’ PCC, is never reported before. The excellent product of HAp was obtained via facile synthesis procedure. Short term reaction and low temperature in the HAp synthesis were the advantages of this procedure. Hopefully through this route, eventually, it can be adopted for industrial scale in production of high purity of HAp with more eficient process.
Material and methods
Materials preparation
The materials were the cockleshells, which was collected from Tembilahan, Indera Giri Hulu, Riau; diammonium hydrogen phosphate ((NH4)2HPO4), Merck); 2M HNO3 solution; ammonium hydroxide (NH4OH) 65% (Merck); CO2 and aquadest. The cockleshells samples were washed and cleaned, then sun dried for two days followed by oven dried at 110oC for 2 h. Dry and clean cockleshells were then crushed and grounded with pulvurizer into the cockleshells powder form.
The formation of PCC
The cockleshells powder was calcinated in the furnace around 5 h at 900°C. The calcined cockle shells was then converted into PCC by carbonation method with the addition of nitric acid solution (HNO3) [1]. The calcined cockle shells (CaO) were mixed into Erlenmeyer flask containing of 2M HNO3 (Merck) solution. The solution was stimulated with constant stirring using magnetic stirrer for approximately 30 minutes, before final filtration. In the meantime, filtrate was heated up to 60oC. The pH of the solution was maintained at scale of 12 by addition of concentrated NH4OH (Merck). The mixture was then filtered again and flowed by CO2 gas until the system reached the pH~8. White precipitate or known as PCC was formed. The PCC was strained and washed several times using distilled water and then dried at 110 °C for 2 h (3). These processes can be represented by the following reactions [2]:
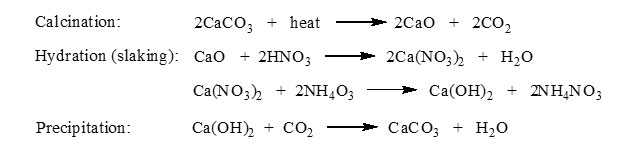
Synthesis of hydroxyapatite
The synthesis of hydroxyapatite from the cockle shells’ PCC was carried out according to Hien et al. [10] by varying the temperature (120, 140, 160 and 180 oC ) and reaction time (14, 16, 18 and 20 h) in the vessel hydrothermal. The PCC was added into (NH4)2HPO4 (Merck) saturated solution forming Ca/P mol ratio of ~1,67 and a little NH4OH (Merck) was added to ensure pH 10 – 11. Sealed vessel was placed in the oven, and the reaction process was carried out in accordance to a suitable variable. The moment reaction was complete, the obtained solids were cleanly washed by distilled water and dried out for 3 h in the oven. The chemical reaction that took place as follows [10].
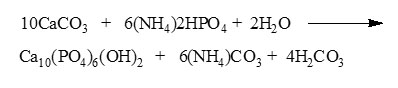
PCC and HAp characterization
The mineralogy of PCC and HAp were characterized using X-ray diffraction (X’Pert Powder DY 3688) with Cu Ka radiation. The crystal structure and phase purity of HAp powder obtained was determined by Rietveld refining of the XRD pattern with the step size and the time per step were 0.01o and 7.14 s respectively.The surface morphology was probed using scanning electron microscopy (SEM) linked to energy dispersive X-ray micro analysis (EDX) (JEOL JED 2300).
The Fourier Transform Infrared Spectroscopy (FT-IR, Perkin Elmer Spectrometer Frontier) was used to analysis bonding structure in the samples. The surface area of the hydroxyapatite were characterized using BET measurements, Surface Area Analyzer (SAA, Quantachrome Nova-Win2).
 |
Figure1: The cockle shells Click here to View figure |
Results and discussion
The synthesis of precipitated calcium carbonated (PCC)was acquired through the modification of the carbonation method, by adding nitric acid on the calcium oxide dissolution process. The comparison of XRD pattern among of raw cockle shells powder, obtained PCC, and the standard calcium carbonate (ICDD – 01-076-2713 (rhombohedral calcite)) are shown in Fig. 2.(a-c). It can be seen that the characteristic peak with the highest intensity of the obtained PCC (Fig.2b) matches with the pattern of calcium carbonate standard namely around 2θ of29.4o and the pattern also clearly shows the PCC is the only phase. The XRD pattern of raw cockle shells (Fig.2a) is different from the calcite standard. The raw cockle shells consist of only aragonite phases. The diffraction peaks at 24.9, 27.0 and 32.8o which are corresponding to the aragonite (Fig.2a). The PCC square form and size of particles that attained in the research were almost uniform (crystallite size 0.6 – 1.0 um). According to the XRD pattern (Fig.2b), it can be concluded that the obtained PCC is the rhombohedral calcite [1,15]. It is in agreement with the SEM morphological image of cockle shells’ PCC displayed in Fig. 3.
 |
Figure2: XRD diffractograms of product: (a) CaCO3 of cockle shells, (b) PCCof cockle shells, (c) calcite, Calcium carbonate standard (ICDD 01-076-2713). Click here to View figure |
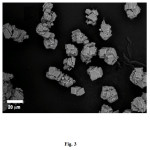 |
Figure3: SEM image of cockle shells’ PCC. Click here to View figure |
HAp manufacture of PCC shells, performed at various reaction times of 14, 16, 18 and 20 h at 180oC. The best results of XRD analysis, was obtained at reaction time 16 h. The XRD patterns of HAp in the optimization of hydrothermal step, where the temperature and the reaction time were varied, are presented in Fig. 4(a-d). Furthermore, the synthesis carried out for 16 h by varying the temperature of 120, 140, 160 and 180oC. The SEM image of HAp synthesized (Fig. 7) corroborates the postulation that the best HAp is achieved when the synthesis process was carried out at temperature of 140 oC and the reaction time of 16 h, where the homogeneous in crystal sizes and the higher purity are obtained.
 |
Figure4: XRD diffractograms for the products of synthesis at varied temperature for 16 h(a) 180oC, (b)160oC, (c) 140oC (d) standard of hydroxyapatite (ICDD-PDF 00-024-0033). Click here to View figure |
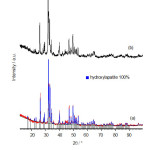 |
Figure5: XRD diffractograms of the hydroxyapatite synthesis at 140oC for 16 h(a) before (b) after calcined at 550oC. Click here to View figure |
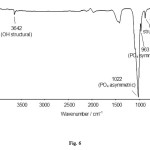 |
Figure6: FTIR spectrum hydroxyapatite of cockle shell sat 140oC for 16 h. Click here to View figure |
Hien et al. [10] reported that the best condition of coral HAp synthesis stood in the temperature of 180oC for 36 h. This situation could be due to the coral particles’ size were in the larger volume (5x5x5 mm) that took a longer time and higher degree of temperature to penetrate diammonium phosphate to form the apatite compound. Unlike in our work, here we used very fine size of PCC particles, so that the HAp were formed at a more convenient condition.
Moreover, an analysis of X-ray diffraction to the compounds of synthesis result on the varied temperature (Fig. 4) with the reaction time for 16 h, is directed in order to analyze the obtained apatite compound. The diffractogram patterns of apatite compound (Fig. 4c) show the similar patterns with the hydroxyapatite standard (ICDD 01-074-9780, Fig.4d) and no other crystalline phases are detected beside this phase. There, it is revealed some peaks with the high intensity that suitable the X-ray hydroxyapatite standard, which characteristic peaks at 2θ values of: 25.9; 31.7; and 32.9 corresponding to (002), (211) and (300) planes.
HAp synthesized at 140oC for 16 h, then calcined at 550oC for 3 h. XRD analysis for both compounds HAp, before and after calcinations, showed the same characteristic peaks (Fig.5a-b). Based on XRD data and Rietveld analysis, the best condition for synthesis of HAp obtained at 140 oC for 16 h. Rietveld analysis of the XRD data HAp synthesized at varied temperature, the purity of HAp synthesized at 140 oC reaches 100% (Fig. 5a). This suggests that, the hydrothermal synthesis method of HAp through the establishment of PCC, is more profitable than synthesis by forming CaO [10].
The FTIR spectroscopy analyses to the HAp of synthesis result on 140oC with time reaction nearby 16 h, it can be seen the specific spectrum patterns for the PO4 adsorption of hydroxyapatite (Fig.6). Meanwhile, in the FTIR spectrum is found the P–O stretching asymmetric adsorption that come from the PO43- on 1150 – 1000 cm–1, which is known as the characteristics of bands for hydroxyapatite and, a medium intensity band at about 960 cm–1 due to symmetric stretching vibration were observed. The bending vibration of PO43- was observed by bands located at 560 – 620 cm-1. In addition, the asymmetric stretching (v) and medium intensity of PO43‒ ion was detected at 1022 and 962 cm–1, respectively. Adsorption bands at 3642 and 631 cm–1 corresponding to OH group and HPO42‒ at 2163 cm–1 [5,10,17]. Adsorption band of free H2O is very weak.
The spectroscopy analysis result using FTIR shows that there is apatite compound marked with the vibration peak of P-O and PO43-. The cluster of PO43‒ with the calcium could be seen with there is no NH cluster adsorption of ammonia or ammonium on the 3150 – 3500 cm–1. The FT-IR analysis shows all typical absorption characteristics of hydroxyapatite. In addition, some carbonate content is also seen (CO32‒ peak around 1400 cm-1), which is an indication of the presence of carbonate apatite or calcium carbonate. This might have originated through the absorption of carbon dioxide from the atmosphere [16].
Fig. 7 shows the SEM analysis of hydroxyapatite compound of cockle shells’ PCC on 140oC for 16 h reaction time. The SEM image displayed the synthesis result of hydroxyapatite compound that has almost the similar form and size. Content of element present in the hydroxyapatite can be confirmed from the EDX data. The result of measurement of elemental composition (Ca and P content) and Ca/P molar ratio are summarized in Table 1. The Ca/P molar ratio of the hydroxyapatite that was synthesized from PCC cockle shell’s about 1.78. The measured Ca/P ratio for this produced powder was higher than stoichiometric ratio (1.67) expected for a pure HA phase. According to the EDX data that shows existence small amounts of aluminium from calcite phase (CaCO3), the source of matter is much more reasonable.
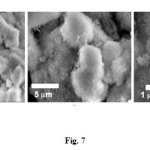 |
Figure7: SEM image of hydroxyapatite compound of cockle shells’ PCC. Click here to View figure |
The isotherm of hydroxyapatite synthesized at 140 oC for 16 h is of type V with a type H-3 hysteresis loop, typical for solids with slit-shaped pores, commonly found with clay materials (Fig. 8). The BET surface area, the total pore volume and the t-plot micropore volume analyses show the following values; 17.8 m2g–1, 0.095 cm3g–1 and -1.559 cm3g–1, respectively. The negative value from t-plot micropore volume exhibited by hydroxyapatiteproved that the material does not contain any micropores.
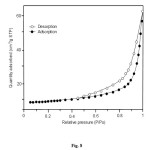 |
Figure8: Nitrogen adsorption–desorption isotherm of hydroxyapatite synthesized at 140 oC for 16 h. Click here to View figure |
Based on those analyses, it can be concluded that the synthesis result of hydroxyapatite at 140 oC during 16 h own hexagonal crystal system, with crystallites size 36-50 nm. The mean grain size of the crystallites of HA particles (D) was calculated using the Scherrer’s equation [18].
Conclusion
The ceramic HAp nanoparticle was successfully synthesized from cockle shell via modified carbonation method followed by the hydrothermal process. The compound analysis of synthesis result, proved that the hydroxyapatite synthesis of cockle shells’ waste, through the formation of PCC produced the excellent result. The shortest time of synthesis and the lower temperature degree, illustrated the advantage of the method in this research. Additionally, the tremendous synthesis HAp at 140 oC for 16 h achieved the hydroxyapatite with the hexagonal crystal structures. As well as the X-ray diffraction patterns and characterization with FTIR are suitable compared with the standard HAp.The dried HAp particles powder was extremely pure with the specific surface area of 17.8 m2g–1.
Acknowledgements
The authors would like to acknowledge the Dirjen Dikti for supporting this research through the Dana Penelitian Desentralisasi.
References
- Jamarun, N.;Yulfitrin, Arief, S. J. Riset Kimia. 2007, 1, 20-24
- Kemperl, J., Macek, J. Int. J. Miner. Process. 2009, 93, 84–88
- Rashidi, N.A.; Mohamed, M.; Yusup, S.;World Acad. Sci. Eng. Technol.2011,60, 818-823
- Riananingsih, J. Thesis, Universitas Andalas , Padang, 2013
- Ishikawa, K. Material. 2010, 3,1138-1155
- Bingöl, O.R.;Durucan, C.; Am.J. Biomed. Sci. 2012, 4, 50-59
- Garcia, B.;Gonzales, G.; Ocanto, F.; Linares, C.F. Indian J. Chem. Technol. 2012, 19, 403-408
- Vazquez-Hernandez, F.; Mendoza-Barrera, C.; Altuzar, V., Melendez-Lira, M.; Santana-Aranda, M.A.; de La, M.;Olvera, L. Mater. Sci. Eng. B. 2010, 174, 290-295
- Tsuchida, T.; Yoshioka, T.; Sakuma, S.; Takeguchi, T.; Ueda, W. Ind. Eng. Chem. Res.2008,47,1443-1452
- Hien, V.D.; Huong, D. Q.; Bich, P.T.N. J. Chem.2010,48, 591-596
- Sanosh, K.P.; Min-Cheol, C.; Balakrishnan, A.; Kim, T.N.; Seong-Jai C. Bull.Mater. Sci. 2009, 32, 465–470
- Sang-Hoon, R. Biomaterials.2002,23,1147–1152
- Saranya, K.; Kowshik, M.; Ramanan, S.R. Bull.Mater. Sci. 2011, 34, 1749–1753
- Strzała, A.; Simka,W.; Marszałek, M. Acta Phys. Pol.2012, 121,561-565
- Kirboga, S.; Oner, M. Chem. Eng. T.2013, 32, 2119
- Eslami, H.; Solati-Hashjin, M.; Tahriri, M. Iran. J. Pharm. Sc.2008, 4, 127-134
- Cimdina, L.B. and Borodajenko, N. Infrared Spectroscopy Materials Science, Engineering and Technology,InTech. Croatia,(2011)
- Cullity, B.D. and Stock, S.R. Elements of X-ray Diffraction, 3rd Edition, Prentice Hall, New-Jersey, (2001)

This work is licensed under a Creative Commons Attribution 4.0 International License.









