Evaluation of Antimicrobial Activity of Some Compounds Isolated from Rhamnus cathartica L.
Manal M. Hamed1*, Laila A. Refahy1, Mohamed S. Abdel-Aziz2
1Medicinal chemistry department, Theodor Bilharz Research Institute, Giza, Egypt
2Department of Microbial Chemistry, National Research Center, Dokki, Cairo, Egypt Corresponding author E-Mail: manalayman90@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/310266
Article Received on :
Article Accepted on :
Article Published : 28 May 2015
A new anthraquinone derivative, 1,8-dihydroxy-2-[(z)-4-methylpenta-1,3-dien-1-yl] anthraquinone (1), together with known anthraquinone compounds were defined to be, 2-acetyl-3,8-dihydroxy-6-methoxy-anthraquinone (2) , emodin (3), glucofrangulin A (4) and phenanthrene derivative named dendrochrysanene (5), lactone compounds named [β-sorigenin (6) and geshoidin (7)], xanthone compound termed pruniflorone H (8), oxanthrone compound named rumejaposide I (9), flavonol compounds called [kaempferol (10) and quercetin (11)] have been isolated from the methanolic extract of Rhamnus cathartica L. All isolated compounds were obtained for the first time from the genus Rhamnus cathartica L. except emodin; they were subjected to antimicrobial activity test; compounds (1), (3), (9) exhibited antibacterial activity [G+ve, G-ve] and anti-yeast activity, whereas, compounds (5), (11) exhibited antibacterial activity [G+ve and G-ve]. But, compounds (2), (4), (7), (10) exhibited antibacterial activity G-ve while compounds (6), (8) exhibited antibacterial activity G-ve and anti-yeast activity. The isolated compounds were confirmed by detailed analysis of their 1H, 13CNMR, mass spectral data and by the results of hydrolytic cleavage.
KEYWORDS:Plant extract; Rhamnus cathartica L.; antimicrobial activity; flavonol
Download this article as:| Copy the following to cite this article: Hamed M. M, Refahy L. A, Abdel-Aziz M. S. Evaluation of Antimicrobial Activity of Some Compounds Isolated from Rhamnus cathartica L. Orient J Chem 2015;31(2). |
| Copy the following to cite this URL: Hamed M. M, Refahy L. A, Abdel-Aziz M. S. Evaluation of Antimicrobial Activity of Some Compounds Isolated from Rhamnus cathartica L. Orient J Chem 2015;31(2). Available from: http://www.orientjchem.org/?p=8864 |
Introduction
Rhamnaceae, is a large family of flowering plants, mostly trees, shrubs and some vines. The family contains 50-60 genera and approximately 870-900 species. Rhamnaceae have a worldwide distribution, but are more common in the subtropical and tropical regions. Economic uses of Rhamnaceae are chiefly as ornamental plants1. The wood of Rhamnus was also the most favored species to make charcoal for use in gun powder before the development of modern propellants. Some Rhamnus species showed insecticidal activity2, anticancer3, antimicrobial4, antioxidant and free radical-scavenging activity5,6. Anthraquninones7-11, anthrones7, flavonoides 5-7,12, anthracene derivatives13, geshoidin, sorigenin glycoside, chrysophanol, physcion, musizin14, emodin10,15, polyphenolic16, procyanidin glycoside17 and essential oil18 are previously isolated from different Rhamnus species. Rhamnus cathartica (buckthorn), is native to Europe, northwest Africa, and western Asia. It is a deciduous shrub or small tree, the seeds and leaves are considered toxic to humans and animals, causing stomach cramps and laxative effects. The chemical compounds responsible for this laxative effect are anthraquinone and emodin, it was effective against various cancer disease tonic, cathartic, diuretic19. Display quotations of over 40 words, or as needed. Microbial diseases such as tuberculosis, candidiasis, cryptococcosis and salmonellosis are examples of known infectious diseases that have been on the increase in the recent history due to HIV/AIDS deadly disease. Resistance to antibiotics such as norfloxacin, ciprofloxacin and amoxicillin clavulanic acid by Pseudomonas aeruginosa and enterohemorrhargic Escherichia coli has been recognized 20. Multidrug resistance poses serious challenges to the medical field and infections caused by multi-resistant bacteria especially in the intensive care units pose a huge problem21. Use of plant products for the control of human diseases has certain advantages besides being cheap to produce; they are biodegradable and readily available. Effective plant extracts can combat human pathogenic bacteria without toxic side effects and environmental hazards22. Our interest in this paper was evaluation the antimicrobial activity of R. cathartica L. extract and the isolated compounds, it also discuss the structural elucidation of the isolated compounds by using different spectroscopic methods.
Experimental
Plant Material
The plant was collected from Giza zoo garden, Egypt at September. The plant was confirmed by Dr. Mohamed El-Gibaly, Lecturer of Taxonomy and Consultant for Central Administration of Plantation and Environment. Voucher specimens were preserved in the Herbarium, Department of Medicinal Chemistry, Theodor Bilharz Research Institute, Giza, Egypt.
Material and Methods
1HNMR analyses were run on JEOL GLM 500 MHz spectrometers relative to TMS in DMSO-d6 and Me2CO-d6. ESI–MS was measured on a Finnigan TSQ 700 GC/MS equipped with a Finnigan electrospray source. Paper chromatography sheets Whattman No.1 (Maidstone, England), the chromatograms were visualized under UV light Vilber Lourmat (VL -6LC France) at 254 and 365 nm. Melting point was detected on (SMDP3 Stuart Scientific UK). Column chromatography was performed using polyamide 6S (Riedel de Darmstadt, Germany) as a stationary phase within a glass column 120 x 8 cm and on Sephadex LH-20 (Pharmacia Fine Chemicals Inc., Uppsala, Sweden). TLC was applied on TLC plates with silica gel 60 F254 (Merck, Darmstadt, Germany). The solvent systems used were CHCl3:MeOH (9:1) (system I), n-BuOH–AcOH–H2O (4:1:5, upper layer) (system II), 15 % aqueous AcOH (system III) and EtOAc:MeOH:H2O (4:3:0.8) (system IV). The authentic flavonoids and sugars were obtained from Department of Medicinal Chemistry, Theodor Bilharz Research Institute (TBRI), Cairo, Egypt.
Equipment and chemicals for antimicrobial assays
Low temperature incubator SHEL-LAB model 2005 sheld on manufacturing.Inc, NUAJARE Biological safety cobient, LABSCO oven Laboratory supply company, Olmon and cokg Germany, Autoclave la Astel lHeorson Germany, Refregerator Toshiba (no frost model FR-GF40P). Nutrient agar medium (LAB M, UK), sucrose (Oxford), NaNO3 (s.d.fine chem. Ltd), MgSO4 (s.d.fine chem. Ltd), KCl (s.d.fine chem. Ltd), FeSO4 (s.d.fine chem. Ltd), K2HPO4 (MERCK), agar-agar bacto (s.d.fine chem. Ltd), Staphylococcus aureus (ATCC 6538-P), Cadida albicans (ATCC 27853), Escherichia coli (ATCC 10231), and Aspergillus niger (NRRL A-326).All the test microbes were obtained from the culture collection at Microbial Chemistry Department, National Research Center.
Extraction and Isolation
The plant material was dried in shade, powdered and subjected to extraction and stored in air tight container for further use. The air-dried, powdered R. cathartica L. (2 kg) were extracted five times with 85% methanol (10 L), under reflux (70 °C) for 7 days, and the combined methanol extract (300 gm) was defatted with n-hexane (2 L), chloroform (3 L), ethyl acetate (2.5 L) then the residual extract was dissolved in deionized water (200 ml), then the salt was removed by adding excess methanol (3 L), and filtered. The filtrate was dried to give (90 gm) extract, that was subjected to polyamide column chromatography (120 X 7 cm, 350 gm) using a consecutive elution with H2O, H2O:MeOH ratio, to pure MeOH elution system which afford 120 fraction. On the bases of comparative paper chromatography (Co-PC) with the use of 1% FeCl3, UV light or Naturstoff spray reagent for detection, the obtained 120 fraction were collected into 6 collective fractions (1 – 6). Fraction 1 (H2O, 17 g) was found to be has no phenolic character with a deep brown color. Fractions 2, 3, 4, 5 and 6 were subjected to further silica gel and Sephadex LH-20 column chromatography, Fr. 2 (3.1 g) was subjected to a H2O:MeOH (90:10) eluent system, and purified by precipitation by ethyl acetate; the latter subjected to Sephadex LH-20 column chromatography with H2O:MeOH (85:15) to afford compound 4 (30 mg) and compound 8 (15 mg). Fr. 3 (3.9 g) was subjected to a column chromatography eluting with H2O:MeOH with increasing ratio from (80 to 20), were then followed by repeated Sephadex LH-20 CC (H2O:MeOH, 90:10) to give compound 3 (23 mg), compound 6 (19 mg) and compound 9 (33 mg). Fr. 4 (1.7 g) eluted with H2O:MeOH (70.5:29.5) and then purified on Sephadex LH-20 CC then acetone to produce compound 1 (29 mg). Fr. 5 (3.9 g) was further isolated by chromatographed on silica gel column eluted with CHCl3:MeOH (9.5: 0.5), then passage over a Sephadex LH-20 column by H2O:MeOH (70:30) elution system and crystallized by ethanol to give compound 2 (31 mg), 5 (28 mg) and 7 (21 mg). Fr. 6 (2 g) was applied to purification with eluent H2O:MeOH (60:40), then subjected to precipitation by acetone followed by crystallization by ethanol to afford compound 10 (17 mg) and 11 (19 mg). Sub fraction 6 contains flavonoids behavior (based on its chromatographic properties of colour change into orange with Naturstoff spray reagent and green with FeCl3).
Acid hydrolysis
Compounds 4, 7 and 9 (about 8.0 mg each) were separately refluxed with 10 mL of 2 M HCl (MeOH:H2O, 1:1) at 100 °C for 4 h. After the MeOH was removed, the residue was diluted with H2O and extracted with CHCl3 (10 mL x 3). The CHCl3 extracts were evaporated under reduced pressure and purified to obtain the aglycone which was identified by comparison with authentic samples, using TLC analysis with solvent system (Benzene: MeOH, 9:1). The aqueous layer was concentrated under vacuum to give a residue of the sugar fraction, then neutralized by NaHCO3. The residue was dissolved in pyridine (0.1 mL) to obtain the sugar moiety which was compared with authentic sugar by using TLC plate with solvent system (EtOAc:MeOH:AcOH:H2O, 12:4:2:3). The comparison with authentic samples showed that the hydrolysates were β-D-glucose, α-L-rhamnose for compound 4 and β-D-glucose for compounds 7 and 9.
Antimicrobial Activity Test
Disc agar plate method was done to evaluate the antimicrobial activity of methanol extract from Rhamnus catharica L. plant and other different isolated pure compounds. The antimicrobial activities of 0.5-cm-diameter filter paper disc saturated with about 1mg of extract and 100 µg of compound sample were tested against four different microbial strains, i.e., Staphylococcus aureus (G+ve bacteria), Escherichia coli (G-ve bacteria), Candida albicans (yeast) and Aspergillus niger (fungi). Both bacterial and yeast test microbes were grown on nutrient agar (DSNZ 1) medium (g/l): beef extract (3), peptone (10), and agar (20). Whereas fungal test microbe was grown on Szapek-Dox (DSMZ130) medium (g/l): sucrose (30), NaNO3 (3), MgSO4.7H2O (0.5), KCl (0.5), FeSO4.7H2O (0.001), K2HPO4 (1) and agar (20). The culture of each microorganism was diluted by sterile distilled water to 107 to 108 CFU/ml to be used as inoculum. 1ml of the previous inoculum was used to inoculate 1l of agar medium (just before solidification) then poured in Petri-dishes (10cm diamter containing 25ml). Discs (5 mm diameter) were placed on the surface of the agar plates previously inoculated with the test microbe and incubated for 24 h for bacteria and yeast but for 48 h for fungus at 37 and 30oC, respectively.
Results and Discussion
The dry leaves of Rhamnus cathartica L. were exhaustively extracted with 85% methanol and defatted, then a chromatographic separation of the defatted methanol extract occurred over polyamide followed by Sephadex LH-20 and repeated silica gel column chromatography resulted in the isolation of a new anthraquinone derivative, 1,8-dihydroxy-2-[(z)-4-methylpenta-1,3-dien-1-yl]anthraquinone (1) (figure 1), together with known anthraquinone compounds were named to be, 2-acetyl-3,8-dihydroxy-6-methoxy- anthraquinone (2)23, emodin (3)24, glucofrangulin A (4)11, one phenanthrene derivative called dendrochrysanene (5)25, two lactone compounds namely [β-sorigenin (6) and geshoidin (7)]24, one xanthone compound termed pruniflorone H (8)26, one oxanthrone compound named rumejaposide I (9)27 and two flavonol compounds identified as [kaempferol (10) and quercetin (11)]28. The structure of these compounds
were elucidated by 1H-NMR, 13CNMR, ESI-MS, IR spectroscopy and by comparison with spectroscopic data reported in literature. Table (2) showed that all isolated compounds [1-11] including the main methanol crude extract have –ve effect against A. niger fungi. All isolated compounds and methanol extract showed significant effect on G+ve bacteria. Bath methanol extract, compounds 1, 3, and 9 showed significant antibacterial effect against Escherichia coli (G-ve), Staphylococcus aureus (G+ve) and antiyeast Candida albicans.
Compound 1 was obtained after further purification of fraction 4 (H2O:MeOH, 70.5:29.5), it is isolated as a deep yellow powder, it gives Rf value 0.57 on paper chromatography in [15 % aqueous AcOH], and its M.P. 268 oC. The preliminary qualitative tests performed by Maobe et al., 2013; indicated that the plant contains anthraquenine29. Physical, chemical and spectroscopic data suggested the anthraquinone of compound 1. IR spectrum of compound 1 exhibited two strong vibration bands at γmax 3444, 3435 cm-1 due to presence of two free hydroxyl groups at C-1 & C-8, two carbonyl bands at γmax 1725, 1680 cm-1 and at γmax 1560 cm-1 for aromatic ring30. 1H NMR spectroscopic data showed two singlet one proton each at δH 12.53, 12.50 due to peri to carbonyl moiety suggested the presence of 9, 10-dioxygenated anthraquinon31,32,33. It also displayed resonance of five aromatic protons at δH 7.53 (1H, d (J=7.5 Hz), H-3), δH 7.94 (1H, d (J=7.5 Hz), H-4), δH 7.97 (1H, d (J=7.5 Hz), H-5), and δH 7.99 (2H, m, H-6 &7) δH 12.50 (OH-8) and δH 12.53 (OH-1)34. 1H NMR spectroscopic data displayed signals suggested presence of methyl penta-1,3-dienyl side chain, three olifinic protons at δH 6.33 (1H, d (J=11.7Hz), H-1′), δH 6.10 (1H, d (J=11.7Hz), H-3′), 6.82 (1H, dd, J=11.7 & 11.6Hz, H-2′) and two methyl singlet at δh 1.61, 1.5435. 13C NMR spectroscopic data [table 1] showed signals for twenty carbon atoms including fourteen aromatic carbon characteristic to anthraquinone compounds36 and six carbons for side chain. 13C NMR spectroscopic data confirmed the presence of two carbonyl groups at δc 190.8 (C-9), 185.0 (C-10), two carbons substituted with hydroxyl groups at δc 160.9 (C-1) and 162.4 (C-8). It showed resonance of four olifinic carbons at δc 119.8 (C1′), 127.7 (C-2′), 122.2 (C-3′), 139.9 (C-4′) and two methyl singlet at δc 26.2 and 18.7. ESI-MS spectrum fragmentation showed a molecular ion peak at m/e 321 [M+H]+ which suggest in addition to previous data that the molecular formula C20H16O4. This data suggest that compound 1 is 1,8 – dihydroxy – 2 – [(z) – 4 – methylpenta -1,3- dien-1-yl] anthraquinone.
Table 1:13CNMR data of compound 1
|
No. |
Compound 1 |
|
1 2 3 4 4a 5 6 7 8 8a 9 9a 10 10a 1′ 2′ 3′ 4′ 5′ 6′ |
160.9 134.2 137.2 120.1 132.2 120.3 135.6 125.1 162.4 118.7 190.8 113.9 185.0 130.6 119.8 127.7 122.2 139.9 26.2 18.7
|
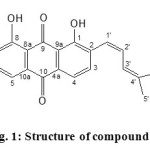 |
Figure1: Structure of compound 1 Click here to View figure |
Table2: Antimicrobial activities of the methanol extract from Rhamnuscathartica L. and its purified compounds
|
Serial no. |
Sample |
Clear zone (ϴmm) |
|||
|
E. coli* |
Staphylococcus aureus ** |
Candida albicans*** |
A. niger |
||
|
Control Crude ext. 1 2 3 4 5 6 7 8 9 10 11 |
Methanol @ Methanol ext. Compd. 1 Compd. 2 Compd. 3 Compd. 4 Compd. 5 Compd. 6 Compd. 7 Compd. 8 Compd. 9 Compd. 10 Compd. 11 |
0 15 15 0 12 0 12 0 0 0 13 0 15 |
0 13 14 13 12 14 14 15 12 15 14 14 12 |
0 16 15 0 11 0 0 12 0 12 14 0 0 |
0 0 0 0 0 0 0 0 0 0 0 0 0 |
* (G-ve); ** (G+ve); ***yeast; @ Control
Structure-activity relationship (SAR)
As the metabolic responsible for antimicrobial activity; Compound 1 showed more antimicrobial activity than the other compounds. Interestingly this compound possesses an additional hydroxyl group at C-8, which might suggest that the structural fragment with a carbonyl and two β-hydroxyls at a linear position in anthraquinones may be an important pharmacophore for the antimicrobial bioactivities37. The bioactive compounds affect the microbial pathogens (test microbes in five main ways depending upon the kind and class of compound, i.e. flavonoid, terpenes, alkaloid etc., these effects are inhibition of cell wall synthesis (most common mechanism), inhibition of protein synthesis (translation) (second largest class), alteration of cell membranes, inhibition of nucleic acid synthesis, antimetabolite activity. Anthtaquinones or quinones are aromatic in nature with two ketone substitution. They are ubiquitous in nature and are highly reactive. It has been investigated that anthraquinones, extracted from different species of Aloe, exhibited antibacterial activity by inhibition of nucleic acid synthesis in the Gram positive bacterium38. In addition, quinones are known to complex with nucleophilic amino acids in proteins often leading to inactivation of protein (including enzymes) and loss of function39. Several investigations have been dealt with the mode of action of flavonoids. The activity of quercetin, for example, has been at least partially attributed to inhibition of DNA gyrase. Mori and colleagues showed that DNA synthesis was strongly inhibited by flavonoids, whilst RNA synthesis was most affected in S. Aureus40. B-ring of the flavonoids may play a role in intercalation or hydrogen bonding with the stacking of nucleic acid bases and that this may explain the inhibitory action on DNA and RNA synthesis40. Ohemeng et al. screened 14 flavonoids of varying structure for inhibitory activity against Escherichia coli DNA gyrase41. It was found that E. coli DNA gyrase was inhibited to different extents by quercetin, enzyme inhibition was limited to those compounds with B-ring hydroxylation41,42.
Conclusions
The methanolic extract of Rhamnus cathartica L. possess antimicrobial activity due to it has high contents of anthraquinone derivatives and flavonols. Chromatographic fractionation afforded eleven compounds belong to anthraquinone, phenanthrene, lactone, xanthone and flavonol. All isolated compounds were subjected to antimicrobial activity test; compounds (1), (3), (9) exhibited antibacterial activity [G+ve, G-ve] and anti-yeast activity, whereas, compounds (5), (11) exhibited antibacterial activity [G+ve and G-ve]. But, compounds (2), (4), (7), (10) exhibited antibacterial activity G-ve while compounds (6), (8) exhibited antibacterial activity G-ve and anti-yeast activity. All compounds include methanol extract give negative effect against A. niger fungi. So we recommended that Rhamnus cathartica L. is suitable for using as antimicrobial plant.
References
- Romani, A.; Zuccaccia, C.; Clement C. An NMR and UV– visible spectroscopic study of the principal colored component of Stil de grain lake Original Research Article. Dyes Pigments 2006, 71, 218-223.
- Ateyyat, M.A.; Abu-Darwish, M.S. Short Communication. Insecticidal activity of different extracts of Rhamnus dispermus (Rhamnaceae) against peach trunk aphid, pterochloroidespersicae (Homoptera :lachnidae). Span. J. Agr. Res. 2009, 7, 160-164.
- Sharma, R. Recommendations on herbs formula in cancer prevention. The open Nutraceuticals J. 2010, 3, 129-140.
- Kosalec, I.; Kremer, D.; Locatelli, M.;Epifano, F.; Genovese, S.; Carlucci, G.; Randić, M.; ZovkoKončić, M. Anthraquinone profile, antioxidant and antimicrobial activity of bark extracts of Rhamnus alaternus, R. fallax, R. intermedia and R. pumila. Food Chem. 2013, 136, 335-341.
- Bhouri, W.; Sghaier, M.; Kilani, S.; Bouhlel, I.; Dijoux-Franca, M.;Ghedira, K.; Ghedira, L.C. Evaluation of antioxidant and antigenotoxic activity of two flavonoids from Rhamnus alaternus L. (Rhamnaceae): Kaempferol 3-O-β-isorhamninoside and rhamnocitrin 3-O-β-isorhamninoside. Food Chem. Toxicol. 2011, 49, 1167-1173.
- Kalidhar, S.B. Reassessment of the structure of an anthraquinone glycoside from Rhamnus formosana. Phytochemistry 1992, 31, 2905-2906.
- Bezabih, M.; Abegas, B.M. Glucofrangulin a diacetate from the fruits of Rhamnus prinoides. Bull. Chem. Soc. Ethiop. 1998, 12, 45-48.
- Abegaz, B.; Dagne, E. Anthracene derivatives of Rhamnus prinoides. Bull. Chem. Soc. Ethiop. 1988, 2, 15-20
- Abergaz, B.M.; Kebede. Geshoidin: Abitter principle of Rhamnus prinoides and other constituents of the leaves. Bull. Chem. Soc. Ethiop. 1995, 9, 107-114.
- Sharp, H.; Latif, Z.; Bartholomew, B.; Thomas, D.; Thomas, B.; Sacker, S.D.; Nash, R.J. Emodin and syringaldehyde from Rhamnus pubesscens (Rhamnaceae). Bioch. Syst. Ecol. 2001, 29, 113-115.
- Marzouk, M.S.; EL-Toumy, S.A.;Merfort, I.; Nawwar, M.A.M. Polyphenolic metabolites of Rhamnus disperma. Phytochemistry, 1999, 52, 943-946.
- Terencio, M.C.; Sanz, M.J.; Paya, M. Hypotensive procyanidin-glycoside from Rhamnus Lycioides ssp. Lycioides. J. Ethnopharmacol. 1990, 30,205-214.
- Gebre, A.; Chandravanshi, B.S. Levels of essential and non-essential metals in Rhamnus prinoides (Gesho) cultivated in Ethiopia. Bull. Chem. Soc. Ethiop. 2012, 26, 329-342.
- Mazzio, E.A.; Soliman, K.A. In vitro screening for the tumoricidal properties of international medicinal herbs. Phytother. Res. 2009, 23, 385-398.
- Bassam, A.S.; Ghaleb, A.; Dahoob, A.S.; Naser, J.; Kamel, A. Antibacterial Activities of some Plant Extracts Utilized in Popular Medicine in Palestine. Turk. J. Boil. 2004, 28, 99-102.
- Ivana, B.S.; Mateus, L.B.P.; Antonio, D.V.; Riad, N.Y. Antibacterial activity of Brazilian Amazon plant extracts. Braz. J. Infect. Dis. 2006, 10, (6).
- Ray, A.B.; Sarma, B.K.; Singh, U.P. Medicinal properties of Plants: Antifungal, Antibacterial and Antiviral Activities. Lucknow, International Book, 2004, 600 pp.
- Baker, R.A.; Tatum, J.H. Novel anthraquinones from stationary cultures of Fusariumoxysporum. J. ferment. Bioeng. 1998, 85, 359-361.
- Zeleke, G. Phytochemical investigation on the stems of Rhamnus prinoides. Addis Ababa University, Science faculty, Department of chemistry 2010, June.
- Yang, L.; Qin, L.; Bligh, S.W.A.; Bashall, A.; Zhang, C.; Zhang, M.;Wanga, Z.; Xub, L. A new phenanthrene with a spirolactone from Dendrobiumchrysanthum and its anti-inflammatory activities.Bioorgan. Med. Chem. 2006, 14, 3496–3501.
- Boonnak, N.; Karalai, C.;Chantrapromma, S.; Ponglimanont, C.; Fun, H.;Kanjana-Opasc, A.; Laphookhieod, S. Bioactive prenylatedxanthones and anthraquinones from Cratoxylum formosum ssp. Pruniflorum. Tetrahedron 2006, 62, 8850–8859.
- Yang, Y.; Yan, Y.; Wei, W.; Luo, J.; Zhang, L.; Zhou, X.; Wang, P.; Yang, Y.; Cheng, Y. Anthraquinone derivatives from Rumex plants and endophytic Aspergillus fumigatus and their effects on diabetic nephropathy. Bioorgan. Med. Chem. Lett. 2013, 23, 3905–3909.
- Singh, D.; Sharma, S.K.; Gupta, H.C.; Sharma, R.A. Isolation and Quantification of Kaempferol-7-O-Glucoside and their Antimicrobial Screening of Cassia nodosa Bunch. Asian J. Biochem. Pharm. Res. 2011, 1, 6-16.
- Maobe, M.A.G.; Gatebe, E.; Gitu, L.; Rotich, H. Preliminary Phytochemical Screening of Eight Selected Medicinal Herbs Used for the Treatment of Diabetes, Malaria and Pneumonia in Kisii Region, Southwest Kenya. Europ. J. Appl. Sci. 2013, 5, 1-6.
- Branco, A.; Pinto, A.C.; Schripsema, J.; Braz-Filho, R. Anthraquinones from the bark of Senna macranthera. An. Acad. Bras. Cienc. 2011, 83, 1159-1163.
- Bilia, A. R.; Yusuf, A. W.; Braca, A.; Keita, A.; Morelli, I. J. Nat. Prod. 2000, 63, 16-21.
- Furumoto, T.; Hoshikuma, A. Dehydroanthrasesamones A and B, anthraquinone derivatives containing a dienyl side-chain from Sesamumindicum hairy roots. Biosci. Biotechnol. Biochem. 2013, 77, 419-421.
- Ling, S.K.; Komorita, A.; Tanaka, T.; Fujioka, T.; Mihashi, K.; Kouno, I. Iridoids and Anthraquinones from the Malaysian Medicinal Plant, Saprosma scortechinii (Rubiaceae). Chem. Pharm. Bull. 2002, 50, 1035-1040.
- Xiang, W.; Song,Q.S.; Zhang, H.J.; Guo, S.P. Antimicrobial anthraquinones from Morinda angustifolia. Fitoterapia 2008, 79, 501-504.
- Levin, H.; Hazenfratz, R.; Friedman, J.; Palevitch, D.; Perl, M. Partial purification and some properties of an antibacterial compound from Aloe vera. Phytother. Res. 2006, 2, 67-69.
- Sher, A. Antimicrobial activity of natural products from medicinal plants. Gomal J. Med. Sci. 2009, 7, 72-78.
- Mori, A.; Nishino, C.; Enoki, N.; Tawata, S. Antibacterial activity and mode of action of plant flavonoids against Proteus vulgaris and Staphylococcus aureus. Phytochemistry, 1987, 26, 2231-4.
- Ohemeng, K.A.; Schwender, C.F.; Fu, K.P.; Barrett, J.F. DNA gyrase inhibitory and antibacterial activity of some flavones (1). Bioorg. Med. Chem. Lett. 1993, 3, 225-30.
- Ammar, R.; Bhouri, W.; Sghaier, M.;Boubaker, J.; Skandrani, I.; Neffati, A.; Bouhlel, I.; Kilani, S.; Mariotte, A.; Chekir-Ghedira, L.; Dijoux-Franca, M.; Ghedira, K. Antioxidant and free radical-scavenging properties of three flavonoids isolated from the leaves of Rhamnus alaternus L. (Rhamnaceae) : A structure-activity relationship study. Food Chem. 2009, 116, 258-264.
- Mai, L.P.; Gueritte, F.; Dumontet, V.; Tri, M.V.; Hill, B.;Thoison, O.; Guenard, D.; Sevenet, T. Cytotoxicity of Rhamnosyl anthraquinones and Rhamnosyl anthrones from Rhamnus nepalensis. J. Nat. Prod. 2001, 64, 1162-1168.
- Kalidhar, S.B.; Sharma, P. Physcion-8-O-gentiobioside from Rhamnus virgata. Phytochemistry 1984, 23, 1196-1197.
- Abegaz, B.M.; Peter, M.G. Emodin and emodinanthronerhamnoside acetates from fruits of Rhamnus prinoides. Phytochemistry 1995, 39, 1411-1414.
- Özi̇pek, M.; Çaliş, I.; Ertan, M.;Rüedi, P. Rhamnetin 3-pcoumaroylrhamninoside from Rhamnus petiolaris. Phytochemistry 1994, 37, 249-253.
- Carmo, M.; Miraglia, M.;Mesquita, A.A.L.; Jesus, M.; Varejão, C.; Gottlieb, O.R.; Gottlieb, H.E. Anthraquinones from Vismia species. Phytochemistry 1981, 20, 2041-2042.
- Tessuer, A.M.; Delaveav, P.; Champion, B. Planta Med. 1981, 41, 337-341.
- Wabo, H.K.; Kouam, S.F.; Krohn, K.; Hussain, H.; Tala, M.F.; Tane, P.; Ree, T.v. ; Hu, Q.; Schulz, B. Prenylated anthraquinones and other constituents from the seeds of Vismia laurentii. Chem. Pharm. Bull. 2007, 55, 1640-1642.
- Hilliard, J.J.; Krause, H.M.; Bernstein, J.I., Fernandez, J.A.; Nguyen, V.; Ohemeng, K.A.; Barrett, J.F.A comparison of active site binding of 4-quinolones and novel flavone gyrase inhibitors to DNA gyrase. Adv. Exp. Med. Biol. 1995, 390, 59-69.

This work is licensed under a Creative Commons Attribution 4.0 International License.









