Development and Validation of RP-HPLC Method for Simultaneous Estimation of Olmesartan and Hydrochlorothiazide in Tablet Dosage Form
Mithun Rudrapal1*, Madhavi Usharani Oduri1, Nageswara Rao Samidala1, B. V. V. S. Surya Kiran2, Julfikar Ali Junejo3, Khumantham Deepak Singh3, Tapash Chakraborty3, Manabendra Debnath4
1Aditya Institute of Pharmaceutical Sciences & Research, Surampalem, E. G. Dist., A. P., India
2Koringa College of Pharmacy, Korangi, Tallarevu (M), E. G. Dist., India
3Department of Pharmaceutical Sciences, Dibrugarh University, Dibrugarh, Assam, India
4Department of Human Physiology, S. Vivekananda Mahavidyalaya, Mohanpur, West Tripura Dist., Tripura, India
Corresponding author: E-mail: rudrapal.m03@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/310236
Article Received on :
Article Accepted on :
Article Published : 29 May 2015
A simple, precise and accurate RP-HPLC method was developed and validated for simultaneous estimation of olmesartan (OLM) and hydrochlorothiazide (HCT) in tablet dosage form. Separation was achieved on a reversed-phase C-18 column (250x4.6 mm i.d., 5µm) using a mobile phase consisting of methanol/acetonitrile (pH 2.6, 70:30, v/v) at a flow rate of 1.0 mL/min and UV detection at 254 nm. The method was validated as per ICH guidelines for linearity, accuracy, precision and robustness. The developed method shows good linearity over the concentration range of 20-80 µg/mL (r2=0.999) for both olmesartan and hydrochlorothiazide. The average percentage recoveries were in the range of 100.0-100.04% and 100.0-100.06% for olmesartan and hydrochlorothiazide, respectively. The limits of detection (LODs) were 0.04 µg/mL and 0.13 µg/mL for olmesartan and hydrochlorothiazide, and limits of quantification (LOQs) were 0.01 µg/mL and 0.05 µg/mL, respectively. Therefore, the proposed method can be applied for routine analysis of the bulk drugs as well as combined pharmaceutical dosage forms of olmesartan and hydrochlorothiazide.
KEYWORDS:Olmesartan; Hydrochlorothiazide; Tablet dosage form; RP-HPLC; Validation
Download this article as:| Copy the following to cite this article: Rudrapal M, Oduri M. U, Samidala N. R, Kiran B. V. V. S. S, Junejo J. A, Singh K. D, Chakraborty T, Debnath M. Development and Validation of RP-HPLC Method for Simultaneous Estimation of Olmesartan and Hydrochlorothiazide in Tablet Dosage Form. Orient J Chem 2015;31(2). |
| Copy the following to cite this URL: Rudrapal M, Oduri M. U, Samidala N. R, Kiran B. V. V. S. S, Junejo J. A, Singh K. D, Chakraborty T, Debnath M. Development and Validation of RP-HPLC Method for Simultaneous Estimation of Olmesartan and Hydrochlorothiazide in Tablet Dosage Form. Orient J Chem 2015;31(2). Available from: http://www.orientjchem.org/?p=8884 |
Introduction
Olmesartan medoxomil (OLM), (5-methyl-2oxo-1,3-dioxol-4-yl)methylester of 4-(1hydroxy-1-methylethyl)-2-propyl-1-{[2′-(1H-tetrazol-5-yl)[1,1′-biphenyl]-4-yl] methyl}-1H-imidazole-5-carboxylic acid (Fig. 1A), is a novel selective angiotensin II type 1 (AT1) receptor antagonist having antihypertensive efficacy. It is an ester prodrug which is completely and rapidly hydrolysed to the active form, olmesartan. It works by blocking the binding of angiotensin II to the AT1 receptors in vascular smooth muscle and as a result of this blockade olmesartan reduces vasoconstriction. This lowers blood pressure by decreasing total peripheral resistance in hypertensive individuals1. Olmesartan medoxomil is obtained as colourless crystalline powder, practically insoluble in water and sparingly soluble in methanol. On the other hand, hydrochlorothiazide (HCT), with the chemical name 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulphonamide-1,1-dioxide (Fig. 1B), is a potent thiazide diuretic, which is widely used in the treatment of hypertension and edema associated with congestive heart failure. HCT inhibits Na+/ Cl– reabsorption from distal renal tubule promoting increased urinary output1,2. It is practically white crystalline powder, very slightly soluble in water, sparingly soluble in methanol and insoluble in ether and chloroform. Despite of having some side effects the combination of OLM and HCT has been proved to be an effective therapeutic alternative for the treatment of hypertension.
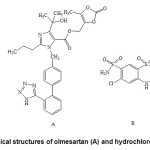 |
Figure1: Chemical structures of olmesartan (A) and hydrochlorothiazide (B) |
The USP describes an RP-HPLC method for the determination of HCT in tablet formulation. Several analytical methods have been reported so far for the determination of HCT in pharmaceutical formulations by polarography, LC, HPTLC and spectrofluorometry. OLM has not yet been officially described in any Pharmacopoeia. Several analytical methods have been reported for its determination in biological sample such as plasma3-6. Literature survey also reveals that a very few analytical methods have been reported for simultaneous determination of HCT and OLM in combined pharmaceutical dosage forms. So, an attempt has been made to develop a simple, precise and accurate method for simultaneous estimation OLM and HCT in tablet dosage form by RP-HPLC.
Materials and Methods
Chemicals
OLM and HCT reference standards were obtained from the Glenmark Pharmaceuticals Ltd., Mumbai, India. Compound olmesartan medoxomil and hydrochlorothiazide tablets (each tablet containing 20 mg of OLM and 12.5 mg of HCT) were procured form the local market. HPLC grade acetonitrile, methanol and water were obtained from Merck Pvt. Ltd., Mumbai, India. Analytical grade di-potassium hydrogen phosphate, triethylamine, phosphoric acid, hydrochloric acid and sodium hydroxide were procured from s.d. Fine Chemicals Ltd., Mumbai, India.
HPLC Instrument and Analytical Conditions
Chromatographic separation was achieved on a Water model 2690 series liquid chromatographic system equipped with a pump, autosampler and a PDA detector. The chromatographic column used in this study was a ThermoHypersil BDS C18 column (250 mm×4.6 mm i.d., 5µm). The column temperature was maintained at 55OC. The mobile phase consisted of methanol and acetonitrile was in a ratio of 70:30, v/v. The pH of the mobile phase was adjusted to 2.6 with phosphate buffer. Isocratic elution was used. The flow rate was 1.0 mL/min and the injection volume was 20 µL. The UV detection wavelength was set at 254 nm.
Standard and Sample Solutions
Phosphate buffer was prepared by dissolving 1.0 g of potassium dihydrogen phosphate in 1000 mL of water, adding 3 mL of triethylamine, and adjusting the pH to 3.0 with dilute phosphoric acid solution.
10 mg of each of OLM RS and HCT RS were individually weighed and transferred separately to a 10 mL volumetric flask. 5 mL of mobile phase was added to the flask and sonicated for 15 minutes. The volume was made up to the mark with the same solvent to get a concentration of 1000 ppm (1.0 mg/mL) for each drug. 1.0 ml of this solution was transferred to another 10 mL volumetric flask and the volume was adjusted up to the mark with mobile phase to obtain a solution containing 100 ppm (100 µg/mL) for each drug.
Twenty OLM-HCT compound tablets were weighed and finely powdered. Powder equivalent to 20 mg of OLM and 12.5 mg of HCT was accurately weighed and transferred to a 100 ml volumetric flask, 50 ml mobile phase was added into it and sonicated for 15 minutes. The volume was made to the mark with mobile phase and filtered through 0.45 µ membrane filter.
Results and Discussion
Method Development
Preliminary studies involved several trial runs using C8 and C18 reversed-phase columns, various mobile phase compositions and different flow rates for the separation of olmesartan and hydrochlorothiazide with good chromatographic parameters (resolution, symmetry, tailing factor etc.). A C18 column (250 mm×4.6 mm i.d., 5µm.) as a stationary phase with a mobile phase consisting of methanol/acetonitrile of pH 2.6 (70:30,v/v) at a flow rate of 1.0 mL/min and a detection wavelength of 254 nm afforded the best separation with well-resolved and sharp peaks for both the drugs. The separation was carried out on an isocratic mode at room temperature and the injection volume was 20 µL.
Method Validation
After method development, validation of the developed method was performed in terms of the following parameters: linearity and range, accuracy and percentage recovery, precision, limit of detection (LOD), limit of quantitation (LOQ), and robustness7-10.
Linearity
The linearity of the method was evaluated by analyzing six (n=6) calibration standards of each of OLM and HCT for a concentration range of 20-80 µg/mL. The calibration curve was constructed by plotting absorbance against concentration of standard drugs. The straight-line equation was determined. The plot of peak area versus concentration was found linear in the range of in the range of 20-80 µg/mL for both OLM and HCT. The regression equations were obtained as follows: y =57311x + 1134 (r2=0.99) for OLM and y = 27958x + 23.07 (r2=0.999) for HCT, where y = peak area, x = concentration of solution; r = the square of determined correlation coefficient. The results implied that the method developed was linear over the specified range.
Accuracy
The accuracy of the method was determined by recovery test. A known amount of each standard powder (with the same proportion as in the drug formulation) was added to blank sample composed of all the excipients equivalent to the ratio of the tablet formulation, which was then mixed, extracted and subsequently diluted to get three different concentrations of each drug (20, 40 and 60 µg/mL for both OLM and HCT).The study was performed three times (n=3) at 50, 100 and 150% concentration levels. The method was found to be accurate with % recovery 100.0-100.04% for OLM and 100.0-100.06% for HCT. Furthermore, the RSD value of the peak areas for each level was calculated, and it was found to be less than 1% at each level for both the drugs. The percentage recovery data are summarized in table 1.
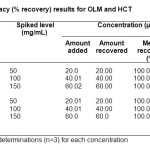 |
Table1: Accuracy (% recovery) results for OLM and HCT Click here to View table |
Precision
Repeatability (intra-day precision) of the method was evaluated by injecting six replicate (n=6) sample solutions of the standard concentration (40 µg/mL, 100%) for each drug on the same day, and the RSD values of the peak areas were calculated, which were found to be 0.008% and 0.01% for OLM and HCT, respectively. The results of repeatability study indicate that the method is repeatable.
Intermediate precision of the method was performed by analysing six samples (n=6) of standard concentration (40µg/mL, 100%) for both OLM and HCT by two analysts in the same laboratory on different day under similar experimental conditions. Results of this study showed that the mean % RSD values for 12 (n=12) samples (6 samples from each analysts) were 0.057% and 0.022% for OLM and HCT, respectively indicating a good intermediate precision of the method.
LOD and LOQ
The limits of detection (LOD) for OLM and HCT corresponding to a signal-to-noise ratio of 3 were 0.04 µg/mL and 0.13 µg/mL, respectively. The limits of quantitation (LOQ) corresponding to a signal-to-noise ratio of 10 were 0.01µg/mL and 0.05µg/mL for OLM and HCT, respectively. The LOD and LOQ indicate that the method is sensitive for simultaneous determination of OLM and HCT.
Stability of Solution and Robustness
The stability of the standard stock solutions (stored at 4oC for two weeks) of each drug was determined at bench top, which showed no significant changes (<2%) relative to freshly prepared standards. The stability of sample solution was tested for every 2 h interval up to 24 h. The RSD values of both OLM and HCT were less than 1.0%.
The robustness of the assay method was investigated by analysing six replicates (n=6) of compound tablets of OLM and HCT by introducing small changes in the chromatographic conditions (in developed method) which included changes of pH of the mobile phase , flow rate and column temperature. The % RSD value of the assay determined under robustness conditions was less than 1.0%, indicating that the developed method was robust. System suitability was determined by six replicate injections of the system suitability solution. The results of system suitability parameters were found to be satisfactory. The results of robustness studies and system suitability parameters are given in table 2 and table 3, respectively.
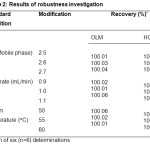 |
Table2: Results of robustness investigation Click here to View table |
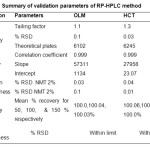 |
Table3: Summary of validation parameters of RP-HPLC method Click here to View table |
Assay of Olmesertan and Hydrochlorothiazide in Tablet Formulation
After successful development and validation of this method, it was employed for analysis of OLM and HCT in compound tablet formulation (Fig. 2). The method results in excellent separation with good resolution between the two analytes. Moreover, the higher percentage of recovery and non-interference of the formulation excipients in retention time of the drugs show the selectivity of the method for estimation of both the drugs in their combined dosage form.
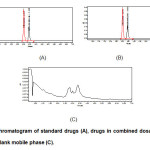 |
Figure2: Chromatogram of standard drugs (A), drugs in combined dosage form (B) and blank mobile phase (C). Click here to View figure |
The mean percentage (n=3) estimated for OLM 100.03% and HCT 100.02% were in good agreement with the label claimed. The mean percentage found and the RSD (Table 4) indicate that the proposed method could be applied for the determination of OLM and HCT in compound tablets.
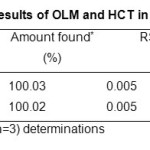 |
Table4: Assay results of OLM and HCT in tablet formulation Click here to View table |
Conclusion
The developed method was successfully applied for simultaneous estimation of olmesertan and hydrochlorothiazide in compound tablet formulation. The proposed method was found to be simple, accurate and precise. The method was free from interferences due to excipients present in formulation. Therefore, this method may be useful for routine analysis of olmesertan and hydrochlorothiazide in bulk drugs and pharmaceutical dosage forms.
Acknowledgment
The authors are thankful to M/s. Active Pharma Labs, Hyderabad for providing necessary laboratory facilities to carry out the work.
References
- K.P. Martindale (Ed.)., The Extra Pharmacopoeia, the Complete Drug Reference, thirty fourth ed. vol. 3, Royal Pharmaceutical Society, 2005.
- British pharmacopoeia: The Stationery Office, London. 2007; 1:1036-1037.
- Fei, L.; Yu, X.; Shu, G.; Z. Jundong, et al. J. Pharm. Biomed. Anal. 2007, 44, 1187-1191.
- Hillaert, S.; Van den Bossche, W. J. Pharm. Biomed. Anal. 2003, 31, 329-339.
- Taomin, H.; Zhong, H.; Bei, Y. J. Pharm. Biomed. Anal. 2006, 41, 644-648.
- Sathe, S.R.; Bari, S.B. Acta Chromatogr. 2007, 19, 270-278.
- Green M., A Practical Guide to Analytical Method Validation, Analytical Chemistry News and Features, May 1, 1996, p. 309A.
- Huber L., Validation of analytical methods, in: Validation and Qualification in the Analytical Laboratories, Interpharm Press, Buffalo Grove, IL, 1998, p. 107.
- ICH, Text on Validation of Analytical Procedures, in: International Conference on Harmonization of Technical Requirements for Registration of Pharmaceutical for Human Use, Geneva, July 2000.
- ICH, Validation of Analytical Procedure: Methodology, in: International Conference on Harmonization of Technical Requirements for Registration of Pharmaceutical for Human Use, Geneva, July 2000

This work is licensed under a Creative Commons Attribution 4.0 International License.









