Destruction of the resins structure due to heating
Bakhtizin Ramil Nazifovich1, Fattakhov Irik Galikhanovich2, Kadyrov Ramzis Rahimovich1, Dilara Ilmirovna Akhmetshina1, Albina Rinatovna Safiullina1
1FSBEI of НРЕ “Ufa State Petroleum Technological University” Republic of Bashkortostan, 450062, Ufa, Kosmonavtov Street, 2FSBEI of НРЕ “Ufa State Petroleum Technological University”, branch, Oktyabrsky Republic of Bashkortostan, 452607, Oktyabrsky, Devon Street, 54а
DOI : http://dx.doi.org/10.13005/ojc/310221
Article Received on :
Article Accepted on :
Article Published : 21 Apr 2015
This article considers a polymer composition based on acetone-formaldehyde (AFR) resin, which is used to reduce water influx into the well bore during the repair insulation work. Pumping into the well bore a composition, based on AFR resin, results in self-heating that causes destruction of its own structure and, therefore, the damage of the polymer blend structure. The purpose of the present article is to find the causes of AFR resin self-heating when injected into the well bore as part of the composite mixture that leads to the destruction of its structure. Author considers exothermic reaction between AFR resin and aqueous solution of sodium hydroxide during the curing of the polymer composition. The features of the given polymer structure are studied as well. The need for further studies of the resin structure is substantiated. Based on the study conducted, five possible reasons of the AFR resin self-heating, leading to destruction of resin structure, are revealed. Recommended practices that may decrease self-heating of the AFR resin are offered.
KEYWORDS:resin; self-heating; mixture; polymer; curing; exothermal reaction; hydrogen bonds; tie points; molecular weight; microheterogeneity; colloidal solutions; crystallization; sodium hydroxide
Download this article as:| Copy the following to cite this article: Nazifovich B. R, Galikhanovich F. I, Rahimovich K. R, Akhmetshina D. I, Safiullina A. R. Destruction of the resins structure due to heating. Orient J Chem 2015;31(2). |
| Copy the following to cite this URL: Nazifovich B. R, Galikhanovich F. I, Rahimovich K. R, Akhmetshina D. I, Safiullina A. R. Destruction of the resins structure due to heating. Orient J Chem 2015;31(2). Available from: http://www.orientjchem.org/?p=8520 |
Introduction
Most of the oil fields in the country are at the final stage of operation. This stage is characterized by the presence of significant number of idle well bores, as well as large water content in the produced products (Oil and gas recovery equipment, 2014).
To reduce watering of produced oil, well bore needs repair insulation work. However, the efficiency of the repair insulation work based on currently existing technology and back fill compounds is not high enough. The reason for the low efficiency is conditioned by use of mixtures, which are based on mineral matters that have astringent property and thus lead to a reduction in reservoir porosity and permeability, as well as lower production output.
In connection with the unresolved problems of the high water content in the oil and inefficient technologies, the most important problem for the repair insulation work is to create a new polymer based backfill mixtures; blocking off water flowing ways by selective action on the cause of flooding, i.e. blanking off nearby reservoirs and watering zones of productive strata, installing water-supply interstratified layers. To achieve these objectives water waterproofing materials are applied.
Main Part
There are two types of water influx localization methods, selective and non-selective, depending on the physical and chemical properties of the material.
Nonselective method consists in the use of a composite material forming a screen, which does not perish in the reservoir for a long time.
This composite material is based on acetone-formaldehyde resin (Buszewski, & Suprynowicz, 1988).
The properties of this material, as well as the mechanism of solid products formation differ from that of mineral backfill solutions. This results in an opportunity to achieve high repair insulation work performance in the well bores.
The advantage of the resin is its low market price, ecological safety and a significant ability to penetrate into the reservoir (Detlefsen, 2002).
The chemical formula of the resin (Paturoev, 1987) is as follows:
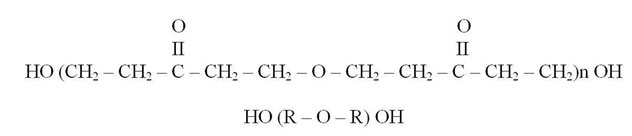
Transition of the resin into a nonfusible and nonsoluble state occurs when appending caustic alkali, in particular, sodium hydroxide (NaOH), into the resin (Korovin, 1998).
Resin curing reaction has the following form (Irzhak et al., 1979):
HO (R – O – R) OH + NaOH = OH (R – O – R) ONa + H2O + q
The sharp rise in the temperature of backfill compound is due to the addition of alkaline solution (NaOH) into the acetone-formaldehyde resin. Since the resin curing reaction is exothermic, it generates heat. This leads to increase in mixture temperature. This is one of the reasons explaining self-heating of the composition mixture, when pumped into the well bore.
Consider other possible reasons for the increase in mixture temperature.
Any cured polymer is heterogeneous at different structure levels. Heterogeneous structure is understood as differences in density fluctuations, as well as the value of adjusted spheres, exceeding the statistical and thermodynamic fluctuations in a given state. There is heterogeneity of the second type, called technological defects (bubbles, surface macro-fissures, etc.), associated usually with the polymer samples preparation technique. When impacting on heterogeneous material, the stress field transforms into a heterogeneous field, generating cumulation of stress around any defect. This leads to local plastic violations and rupture of the bonds between atoms; in addition, most intense bonds rupture first. Separation of bonds occurs due to deviation of the thermal motion energy, while mechanical stress, which also applies at this moment, lowers the potentials barrier, which must be overcome to separate the atoms. Cumulation of the required number of ruptures causes the formation of submicroscopic cracks (Irzhak, 1979).
Besides, the expanded conformation of the polymer, i.e. increase in weight and volume due to the absorbed liquid is also possible (Kireev, 1970). An increase in molecular weight of the polymer contributes to an increase in its temperature. Thus, it can be assumed that this is another reason causing self-heating of the resin.
The initial stage of the AFR resin curing, after adding curing promoter NaOH, is followed by formation of hydrogen bonds between the molecules, as the amount of alkali increases (Darly et al., 2010). This, probably, occurs due to the fact that in the presence of a strong alkali the proportion of carbonyl groups becomes diol. The resulting hydroxyl groups form the hydrogen bonds with the oxygen atom of the remaining functional groups >C=O, which have concentrated quite large negative charge. The AFR curing process involves carbonyl group (Kuznetsova, 2008).
Maybe, it is associated with the presence of aldol condensation at the AFR curing (Fig.1).
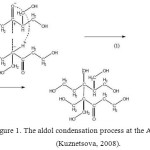 |
Figure1: The aldol condensation process at the AFR curing(Kuznetsova, 2008). Click here to View figure |
Despite the relatively low energy of each individual H-bond, their interaction throughout the polymer molecule leads to strong intermolecular interactions that result in raising the compound temperature.
Morphological qualities of the most polymers depend on the curing process. Certainly, the ability of polymers to form higher configuration regularities is associated with the geometry of the primary supermolecular feature.
In the context of polymer, the word “structure” must be divided with regard to various scale criteria. In general cases, we separate the internal structure of the chains and the external structural organization of the chains. It is appropriate to ascribe external structure to the supermolecular one. It covers all levels of the structures in polymers having a size greater than or equal to the size of macromolecules, i.e. elementary supermolecular structures, which may be represented by single ordered areas. In real polymers, due to imperfect stereoregularity or kinetic and thermodynamic conditions, elements with varying degrees of regularity appear (Hasmukh et al., 2010).
Elements of supermolecular structure have different names, though the most frequently used names are domain, globule, and crystallite. Its main feature is the one-dimensionality of the chains orientation within a domain and random orientation at the boundary. These structural formations are found in both crystalline and amorphous polymers. Therefore, their properties can be described as a single model (Lipatov, 1984).
It is known that the most systems, based on high molecular weight compounds, are characterized by phase separation of the components. Chemical linkage of components, which do not react chemically, can stop this process. Properties of the systems are directly dependent on the distribution of micro- heterogeneities (Gel’fman, 2004).
Microheterogeneities of polymer systems give every reason to regard them as colloidal systems.
A highly developed interphase results in excess of surface free energy; thus the system is unstable in terms of its thermodynamics and aggregation. This also may cause heating of the composite mixture (Pismenko, 2003).
Production of cross-linked polymers by polycondensation out of oligomers, which may crystallize, results in the formation of the polymer, which also has the ability to crystallize. However, the degree of crystallinity of cross-linked polymer is always lower than that of the original oligomer. At that, the higher the concentration of lattice points, the higher the degree of crystallinity.
Since the crystallization makes for temperature increase, it also can be regarded as one of the causes of the mixture’s heating.
Chemical lattice points in cross-linked polymers make significant adjustments to the ability of creating long-range order. These adjustments increase with increasing the degree of disorder in oligomers during the polymer formation process (Rostiashvili, 1987).
Research dealing with the effect of the number and nature of the distributions, the number of cycles of different sizes and structures on the morphology of the polymers would have become very useful, because they would allow purposefully manage this impact even at the stage of polymer production.
Increasing the concentration of the chemical tie points upstages regulation of morphological properties of the polymers, making it virtually impossible.
Increasing the concentration of chemical and physical tie points contributes to so strong shift of defrost temperature of segments mobility towards the high temperature area that it is almost impossible to detect them, since thermal decomposition of the polymers occurs that results in destruction of the original structural organization. Therefore, here all boundaries, which distinguish physical tie points from chemical ones, are eliminated (Rozenberg, & Irzhak, 1981).
The properties of composite materials and coatings are largely conditioned by the interfacial layer structure and the properties. The interfacial interactions, which affect the layer structure, occur exactly in the interfacial layer. Layer structure, in turn, determines the nature of the interfacial interactions and thus the strength of interfacial relationships, as well as properties of the composite material.
Numerous experiments have revealed that the interfacial layers may have a large thickness, often equal to several microns (Rozenberg, & Irzhak, 1981; Glotovo et al., 1982; Manson, & Sperling, 1979).
Studies of recent years have enabled to detail the internal stress mechanism in the case, where the cross-linked polymers are formed by polycondensation, i.e. when mechanical destruction of the cross-linking occurs in a synthesis process affecting by boundary stresses. It was proved in many studies (Mozninea et al., 1998; Carlssona et al., 2013) that during the formation of a cross-linked structure, stress gradient arises at the interphase. Occurrence of internal stresses in the system is apparently the consequence of chemical shrinkage of curable polymer. Note also that since the reaction rate is high, a strain rate at the interphase is relatively large as well. This leads to a modification of the cross-linked structure.
Since the interfacial stresses have macroscopic scale regardless of the specific mechanism of surface layer modification as affected by the force field, the length of the modified layer will also have a macroscopic scale.
Formation of a surface layer with higher density should lead to the fact that its structure will not be identical to that of the polymer bulk. At that, excessive density in the surface layers of the polymer can only be implemented due to higher regularity, comparing with that in the bulk, since many polymers anyway are characterized by relatively high packing factor (Greinke, 1984).
On the basis of the data studied, we revealed the following five possible reasons for resins self-heating:
- exothermic reaction of resin curing;
- increase in molecular weight due to expanded conformation;
- formation of intermolecular hydrogen bonds;
- colloidal nature of the system;
- polymer crystallization.
Results
The self-heating of the composition results in the destruction of the polymer structure and the occurrence of significant stresses. Especially essential stresses occur upon cooling that leads to cracking of the product.
We can suggest the following possible solutions to this problem.
- Use of active filler.
The AFR resin is designed for the preparation of mixtures that are exploited under the effects of water.
The tolerance of the polymer to corrosive environments is determined primarily by the resin parameters and the physical and chemical nature of fillers. (Hasmukh et al., 2001).
The AFR resin is an aqueous solution of low molecular weight products of formaldehyde polycondensation. The water content in the resin ranges within 15-35%. This water, chemically bound in the resin molecule, begins to escape during the curing process that may lead to a weakening of the “polymer-filler” adhesive bond and adversely affect the resistance of polymer-impregnated concrete.
Effect of physically active agents consists in their penetration into the polymer-impregnated concrete and the weakening of the bond between resin macromolecules, as well as adhesive strength with filler, loosening the structure and leaching of soluble substances from the system. The resin structure changes when exposed to chemically hostile environment. With regard to the AFR resin, water is an aggressive medium from both physical and chemical viewpoints. Water chemically reacts with the resin that leads to significant structural changes in the AFR resin, containing bonds, which can be easily hydrolyzed. The latters are decomposed in water, their molecular weight decreases and mechanical properties deteriorate (Takhirov, 1988).
Sufficient stability of the resin is achieved by the application of the active filler, which is capable of forming a strong adhesive bond in the contact area and undergo the reaction of hydration with the water, released from the system.
The phenolic alcohol is effective AFR resin filler (Paturoev, 1987).
The appropriate maximal concentration of phenolic alcohol is 10-20% by polymer weight.
The increase of phenolic alcohol concentration up to 20% leads to an increase in strength of the mixture due to the interaction of hydroxyl groups of the resin with hydrogen phenol nucleus (Takhirov, 1988).
2. Adding flexibilizers.
Flexibilizers are added to polymer to extend the range of operating temperatures (to increase high-elasticity qualities and reduce brittleness).
Flexibilizers are substances, whose purpose is to reduce the interaction of molecules to the desired degree, or to provide the uniform distribution of the modifying parts in the polymer.
Application of flexibilazers results in the decrease of viscosity, the vitrifaction temperature and brittleness in the glassy state; though, at the same time, this leads to decrease in polymer strength, increase of the linear thermal expansion rate and, consequently, the risk of increasing deformations.
Reducing the temperature, at which the polymer starts to vitrify, results in expanding the temperature limits, in which the resin may be in the rubber-like state, as well as in increasing the resistance to frost.
Reducing the viscosity of the mixture is especially important, because the preparation of components out of thermoplastic material can limit the viscosity from 102 to 103. In order to achieve such viscosities without the use of flexibilazers, one has to increase the mixture temperature that will result in thermal and thermo-oxidative degradation. Flexibilazers, used in the mixture, decrease viscosity of the mixture, lower the temperature needed for preparation, and essentially reduce or even eliminate the degradation (Gottlieb, 2008).
3. Preparation of a mixture out of two or more polymers.
Various polyalloys of two or more polymers are often used for obtaining specific physical and mechanical properties.
Adding not more than 10% of one polymer into another can significantly change the properties of the system.
Basically, polymers do not mix with each other (i.e., polymers are incompatible) that prevents us from obtaining a true homogeneous mixture. There are many ways to modify and adjust polymer properties (Chernin et al., 1982).
Though, mostly low adhesion characteristics result in low mechanical properties of the polymers. Low adhesion of polymer surfaces occurs when polymers do not interact with each other specifically.
Therefore, mixtures of different polymers must possess good adhesion. Necessary adhesion is achieved by adding to the mixture a compatibilizer, which combines polymers (Kakhramanly, 2013).
4. Use of sodium hydroxide, dissolved in water.
In (Kadyrov et al., 2013) the authors propose a new method of preparing a composition mixture, based on mixing of the AFR resin with sodium hydroxide and water to solve the technical problem: increasing efficiency and, at the same time, quality of repair insulation work along with a decrease in the effect of heat release during the exothermic reaction, when preparing and strengthening the cured binding composition.
First, sodium hydroxide is mixed with water. The resulting solution is divided into two equal portions. One portion is added into the AFR and stirred until the mixture becomes homogeneous. This mixture is aged during the period from 60 to 120 minutes. Then the next portion of solution is added into the mixture while stirring. After this, homogeneous mixture is applied into the well bore.
The AFR curing reaction proceeds with heat release. Therefore, it is necessary to reduce the reaction effect on the curing process of the composite mixture.
Batch-wise application of sodium hydroxide solution into the AFR resin in equal proportions and further aging of the mixture after adding to the resin of the first portion of dissolved sodium hydroxide, makes it possible to reduce the effect of released heat, and contributes to produce cementing stone throughout the technologically required period in order to carry out repair insulation work , and thus, to eliminate the emergency situations when preparing , introducing into the well bore and squeezing the cementing mixture. If the solution is aged during the time less than 60 minutes, than the effect of heat release during the polycondensation is not eliminated, whereas aging time exceeding 120 minutes leads to increase of the cost of repair insulation work that is economically untenable.
Preparation of backfill mixture in the proposed sequence enables to obtain cementing composition with controlled curing period varying from 75 to 405 minutes. Application of sodium hydroxide, dissolved in water, into the AFR resulted in a measured increase in temperature of cementing mixture as compared to the cementing mixture, produced based on conventional method. This enables us to produce cementing mixture, apply it into the well bore and burst throughout the entire technologically required time period. In addition, a slight adjustment of time, required for curing of cementing mixture after increasing its volume from 0.0005 to 0.002 m3 indicates the reduction of the exothermic heat effect.
The presence of the AFR resin at the concentration of 80-95% by weight in the cementing mixture in comparison with the cementing mixture produced conventionally, contributes to formation of cementing stone-solution having high persistent quality. After 28 days of exposure of cured specimens by stratum water, the measured compressive strength was 12.21-16 52 MPa, and the strength of the sample was 0.89-8.87 MPa. Studies have revealed that after a long storage of produced cured cementing compositions in stratum water their sizes do not change, i.e. there was no shrinkage (Kadyrov et al., 2011).
Therefore, the goal of the current work, consisting in increase of efficiency and the quality of repair insulation work, is achieved by adjusting the curing time of the cementing mixture and simultaneous decrease in the effect of heat release during the exothermic reaction taking place when preparing and increasing the strength of the cured cementing composition.
Final Part
Thus, based on studies conducted, we can identify five reasons for self-heating of the resin:
- Adding alkali solution (NaOH) to the AFR resin leads to a sharp increase in the temperature of backfill compound. Curing of the resin is an exothermic reaction and, consequently, results in heat release that increases the temperature of the mixture.
- The absorption of liquid results in increased mass and volume. This contributes to an increase in polymer temperature and can be one of the reasons of the resin self-heating.
- The beginning of the AFR resin curing after adding NaOH as a curing promoter, while increasing the amount of alkali, is followed by formation of hydrogen bonds between molecules. The resulting hydroxyl groups form the hydrogen bonds to the oxygen atom of the remaining functional groups > C=O, which concentrate quite large negative charge. Carbonyl group is involved in the AFR curing process. Despite the relatively low energy of each individual H-bond, their interaction throughout the polymer molecule leads to a strong intermolecular interaction that results in an increase of the composite temperature.
- A highly developed interphase results in excess of surface free energy; thus the system is unstable in terms of its thermodynamics and aggregation. This also may cause heating of the composite mixture.
- Production of cross-linked polymers by polycondensation out of oligomers, which may crystallize, results in the formation of the polymer, which also has the ability to crystallize. Since the crystallization makes for temperature increase, it also can be regarded as one of the causes of the mixture’s heating.
Naturally, we have identified just a small part of the factors leading to self-heating of the resin that takes place in the industry, such as oil production. We hope that the material presented in the article will be useful to oil and gas production workers.
Conclusions
We carried out a comprehensive study of a method to insulate well bore against water influx using a new preparation method of insulating mixture (Kadyrov et al., 2013).
For comparison, we present the two other well-known methods for preparation of an insulating mixture.
There is a method to limit the water influx into the well bore, which concludes in the injection of a mixture consisting of the urea-formaldehyde or AFR resin (polymerization process promoter), water, additives and fillers. After bursting a composition into the well bore, it is aged until curing and transforming into a stone.
Disadvantages of this method are as follows:
- complexity of the mixture preparation at the site of repair insulation work , since this needs a significant amount of specific equipment;
- displacing fluid can dilute the injected insulating composition (i.e. change the concentration of the injected material) that reduces the curing effect and increases curing time.
There is also another known method, which is as follows.
Urea is applied into the AFR resin, which is heated preliminary up to 50-600C. After the mixture is cooled, water and alkali curing agent are added. The composition is stirred for fifteen minutes.
Disadvantages:
- adding the alkali increases the mixture temperature that leads to an increase in exothermic effect on the mixture curing process;
- curing time control becomes difficult.
- difficulty in preparing mixture at the site of repair insulation work, since the resin must be preheated.
Studies conducted in (Kadyrov et al., 2013) suggest that the proposed method is devoid of above disadvantages.
The effectiveness of the proposed method consists in the following:
- it allows one to control the curing time of the cementing mixture;
- it makes possible to decrease the effect of heat release during the exothermic reaction, when preparing the composition;
- it provides the increased strength of the cured cementing stone.
These factors result in enhanced efficiency and quality of repair insulation work.
Thus, in this work we have achieved essential positive improvements in preventing water influx into the well bore.
References
- Buszewski, B., & Suprynowicz,. Chemical Papers, . 1988. 42, 835-840.
- Carlsson, L., Adams D., & Pipes R. Polymer Reviews, 2013.. 53(2), 277-302.
- Chernin, I., Smekhov, F., & Zherdev Yu. (1982). Epoxy polymers and compositions. Moscow: Chemistry.
- Darly, R., Pompeua, F., Mourab, G., Silvab, E., & Rogezb, H. Separation Science and Technology, 2010. 45(5), 700-709.
- Detlefsen, W. Phenolic resins:. Adhesion Science and Engineering, 2002. 1, 869-945.
- Gel’fman, M., Kavalevich, O., & Yustratov, V. (2004). Colloid chemistry (2nd ed.). St.-Petersburg: Lan Publisher.
- Glotova, Yu., Ponomareva T., Shteinberg V. et al. 1982., 24(5).
- Greinke, R. Carbon, 1984. , 22(2), 228.
- Gotlib, E. (2008). Plasticization of polar rubbers, linear and cross-linked polymers. Kazan: Kazan Technological University Press.
- Irzhak, V., Rozentberg, B., & Enikolopyan, N. (1979). The cross-linked polymers: synthesis, structure, properties. Moscow: Nauka.
- Kakhramanly, Yu. (2013). Incompatible resin mixtures and related composite materials. Baku, ELM.
- Kadyrov, R., Sahapova, A., Hasanova, D. Zhirkeev, A., Patlay, A., & Fattakhov, I. (2013). Preparation method of backfill compound for repair insulation work. Patent #RU 2485285 of the Russian Federation.
- Kireev, V. (1970). A short course of physical chemistry (4th ed.). Moscow: Chemistry.
- Korovin, N. (1998). General chemistry: Textbook. Moscow: Higher School.
- Kuznetsova, O., & Arhireev, V.. Bulletin of Kazan Technological University, 2008. 5, 90-94.
- Lipatov, Yu. (1984). Colloid chemistry of polymers. Kiev: Naukova Dumka.
- Manson., G., & Sparling, L. (1979). Polymer blends and composites. Moscow: Chemistry.
- Mozninea, R., Blanca, F., Lieutiera, M., & Leforta A. The European Physical Journal Applied Physics. 1998. 2(2), 127-134.
- Oil and gas recovery equipment (2014, January 10). Retrieved October 15, 2014 from http://neftrussia.ru/
- Patel, H., & Patel, B. International Journal of Polymeric Materials and Polymeric Biomaterials, 2010. 59(3), 151-160.
- Paturoev, V. (1987). Polymer-impregnated concretes. Moscow: Stroyizdat.
- Patel, H., Dixit, B., & Dixit, R. Polymer-Plastics Technology and Engineering, 2001.. 40(1), 53-63.
- Pis’menko, V. (2003). Disperse systems. Part 1. Molecular disperse systems (regular solutions). Ulyanovsk: State Technical University (UlSTU).
- Rostiashvili, V., Irzhak, V., & Rosenberg V. (1987). Vitrifaction of polymers. Leningrad: Chemistry.
- Rozenberg, B., & Irzhak, V. (1981). Proceedings of the 5th All-Union Conference on composition materials. Moscow: Moscow State University (MGU).
- Takhirov, M. (1988). Concretes with the addition of acetone-formaldehyde resins. Moscow: Stroyizdat.

This work is licensed under a Creative Commons Attribution 4.0 International License.









