Design, synthesis and anticancer activity of new 3-cyano-2 (1H) -pyridone and 3-cyanopyridine-2-(1H)-thione derivatives
E. A. Abdel Motaal1,2, M. A. Salem*3,4, M. H. Helal1,3, M. S. A. El-Gaby5
1Department of Chemistry, Faculty of Arts and Science, Northern Border University, Rafha, KSA; 2Fats and Oils Department, National Research Centre, Cairo, Egypt. 3Department of Chemistry, Faculty of Science and Arts, King Khalid University, Mohail Assir, KSA; 4Department of Chemistry, Faculty of Science, Al-Azhar University, 11284 Nasr City, Cairo, Egypt; 5Department of Chemistry, Faculty of Science, Al-Azhar University at Assiut, Assiut71524, Egypt; m_eltayyeb@hotmail.com
DOI : http://dx.doi.org/10.13005/ojc/310230
Article Received on :
Article Accepted on :
Article Published : 05 Jun 2015
The main objective of the present research study is to synthesize some novel chalcone, cyanoacetohydrazone, enaminone, 3-cyano-2(1H)-pyridone and 3-cyanopyridine-2-(1H)-thione derivatives and evaluate them for their anticancer effect. The novel chalcones 2a-c were achieved by Claisen-Schmidt condensation of appropriate benzaldehydes with ethanone derivative 1. Treatment of cyanoacetic acid hydrazide with ethanone derivative 1 yielded the correspondinghydrazone derivative 3. Condensation of ethanone derivative 1 with DMF-DMA afforded (E)-3-(dimethylamino)-1- (4- morpholinophenyl)prop-2-en-1-one 4. Heterocyclization of chalcones 2a-c with cyanothioacetamide yielded 2-thioxo-1,2-dihydropyridine-3-carbonitriles 7a-c. In a similar manner, cyclocondensation of chalcones 2a,b with cyanoacetamide afforded the corresponding 2-oxo-1,2-dihydropyridine-3-carbonitriles 8a,b. The Reaction of compound 2a with ethyl cyanoacetate furnished 2-oxo-1,2-dihydropyridine-3-carboxylate 12. The 2-oxo-4-phenyl-1,2-dihydro-pyridine-3,5-dicarbonitriles 14a,b were obtained by cyclization of cyano-acetohydrazone 3 with cinnamonitriles. The structures of the synthesized compounds were confirmed by elemental analysis, mass spectrometry, IR and 1H-NMR spectroscopy. The anticancer activity of the newly synthesized compounds was screened in vitro against Human lung carcinoma (A 549) cell line indicating that compounds 7b and 8a possess the most potent inhibitory effect against the human lung carcinoma cell line (A549).
KEYWORDS:Chalcones; Cyanoacetohydrazone; 3-cyano-2 (1H)-pyridones; 3-cyanopyridine-2-(1H)-thiones; Anticancer activity
Download this article as:| Copy the following to cite this article: Motaal E. A. A, Salem M. A, Helal M. H, El-Gaby M. S. A. Design, synthesis and anticancer activity of new 3-cyano-2 (1H) -pyridone and 3-cyanopyridine-2-(1H)-thione derivatives. Orient J Chem 2015;31(2). |
| Copy the following to cite this URL: Motaal E. A. A, Salem M. A, Helal M. H, El-Gaby M. S. A. Design, synthesis and anticancer activity of new 3-cyano-2 (1H) -pyridone and 3-cyanopyridine-2-(1H)-thione derivatives. Available from: http://www.orientjchem.org/?p=9052 |
Introduction
The pyridine skeleton is of great importance to chemists as well as to biologists as it is found in a large variety of naturally occurring compounds and also in clinically useful molecules having diverse biological activities [1-4]. Cyanopyridines are important intermediates in the pharmaceutical industry for the synthesis of nicotinamide, nicotinic acid and isonicotinic acid [5,6].The importance of cyanopyridines in organic synthesis has increased over the past few decades because they are among the most versatile organic synthetic intermediates [7-11] .The 3-cyanopyridin-2-one nucleus is the structural basis of the alkaloid Ricinine (A), the first known alkaloid containing a cyano group. Milrinone (B) is a 3-cyano-2-oxopyridine derivative that has been introduced to the clinic for the treatment of congestive heart failure [12,13], Figure 1. Cheney and co-workers reported 4,6-diaryl-2-oxo-1,2-dihydropyridine-3-carbonitriles as inhibitors of the oncogenic serine/threonine kinase PIM-1, which plays a role in cancer cell survival [14].3-Cyano-2-thioxopyridines are of a great interest due to their synthetic capabilities and also due to the wide spectrum of biological activity [15]. Synthetic chalcone [16] and hydrazone [17] derivatives were reported to have anticancer activity. Prompted by the above facts and in continuation of our efforts in the field of biologically active heterocyclic compounds [18-25], we hereby report the synthesis and anticancer evaluation of new chalcone, cyanoacetohydrazone, enaminone, 3-cyano-2(1H)-pyridone and 3-cyanopyridine-2-(1H)-thione derivatives containing morpholine moiety.
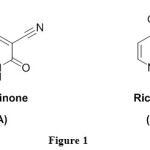 |
Figure 1 Click here to View figure |
Results and discussion
Chemistry
The key intermediate 1-(4-morphlinophenyl) ethanone 1 was obtained by nucleophilic displacement of 4-flouroacetophenone with morpholine in aprotic solvent in the presence of anhydrous potassium carbonate as a base under reflux [26] (Scheme 1).
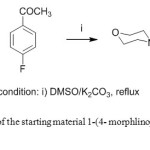 |
Scheme1: Synthesis of the starting material 1-(4- morphlinophenyl) ethanone derivative 1. Click here to View scheme |
The Claisen-Schmidt condensation of appropriate benzaldehydes with ethanone derivative 1 in ethanol in the presence of sodium hydroxide as a base catalyst at room temperature afforded the novel chalcones 2a-c in good yields (Scheme 2). The structures 2a-c were established on the basis of their analytical and spectral data. The infrared spectra of 2a-c in general showed absorption bands at 2972-2848 cm-1 region due to CH-aliph., 1647-1648 cm-1 due to the carbonyl and 1607-1594cm-1 due to ethylenic bond functions. The 1HNMR spectra (DMSO-d6) of the synthesized compounds 2a-c revealed two doublets at 6.96-8.20 ppm region with a characteristic coupling constant 15-16 Hz, which confirms the formation of a,b-unsaturated carbonyls. The values of spin interaction constant J (15-16 Hz) [27] corresponds to an E-configuration on the double bond. The mass spectrum of compound 2a showed a molecular ion peak at m/z = 293 which is the base peak in the spectrum and corresponding to the molecular formula C19H19NO2, Chart I. Also, the mass spectrum of compound 2c was compatible with the molecular formula C23H26N2O3 (m/z = 378). The reaction of cyanoacetic acid hydrazide with ethanone 1 by refluxing in ethanol yielded hydrazone derivative 3. The structure of compound 3 was confirmed on the basis of its elemental analysis and spectral data. The infrared spectrum of the reaction product showed the characteristic absorption bands at 3347 cm-1 for the NH group, 2977, 2842 cm-1 for the CH-aliph, 2260 cm-1 for C≡N group and 1660 cm-1 for the C=O group. The 1HNMR spectrum (DMSO-d6) of the reaction product, which showed two triplets at δ 3.26, 3.71 ppm assigned to the morphonyl protons, singlet signal at δ 4.35 ppm assigned to the methylene protons and two doublets at δ 6.96 , 7.80 ppm assigned to AB-system in addition to the presence of downfield singlet at δ 9.27 ppm assigned to the NH proton. Treatment of ethanone derivative 1 with N,N-dimethylformamide-dimethylacetal (DMF-DMA) in dry m-xylene at reflux temperature, afforded a yellow crystalline product identified as (E)-3-(dimethyl-amino)-1-(4-morpholinophenyl)prop-2-en-1-one 4. The infrared spectrum of the reaction product showed the characteristic absorption bands at 3041, 2968, 2886, 2852 cm-1 for the CH-arom., and CH-aliph. and 1636 cm-1 for the C=O group in addition to the presence of ethylenic bond at 1601 cm-1. The 1HNMR spectrum (DMSO-d6) showed a broad singlet at δ 3.19 ppm assigned to the N,N-dimethyl protons, two triplet at δ 3.22, 3.75 ppm assigned to the morphonyl protons and two doublets at 5.76, 7.60 ppm assigned to the ethylenic protons in addition to the presence of two doublets at 6.80, 7.83 ppm assigned to the aromatic protons. The value of the coupling constant (J =12.5 Hz) [28] for the ethylenic protons indicates that the enaminone 4 exists in the E-configuration. The mass spectrum of compound 4 showed a molecular ion peak at m/z = 260 (94.74%) corresponding to the molecular formula C15H20N2O2.The base peak was found in the spectrum at m/z = 243 (M- NH3) (Scheme 2).
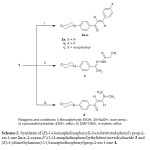 |
Scheme2: Synthesis of (E)-1-(4-morpholinophenyl)-3-(substituted-phenyl) prop-2-en-1-one 2a-c, 2-cyano-N’-(1-(4-morpholinophenyl)ethylidene)acetohydrazide 3 and (E)-3-(dimethylamino)-1-(4-morpholinophenyl)prop-2-en-1-one 4. Click here to View scheme |
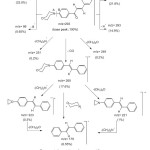 |
Chart 1 Click here to View chart |
The chalcones are important and versatile reagent which has especially been used for the synthesis of polyfunctionalized heterocycles [29]. The reactivity of the chalcones 2 towards some carbon nucleophiles was investigated. The reaction of the chalcones 2a-c with cyanothioacetamide in refluxing ethanol containing catalytic amounts of piperidine yielded 2-thioxo-1, 2-dihydropyridine -3-carbonitriles 7a-c rather than compound 6 (Scheme 3). The structures of compounds 7a-c were established on the basis of their elemental analysis and spectral data. The infrared spectra of compounds 7a-c displayed absorption bands for NH, CH-aliph, and C≡N functions and showed the lake of absorption band corresponding to a carbonyl function. The 1HNMR spectrum (DMSO-d6) of compound 7a revealed two triplets at δ 3.29, 3.74 ppm assigned to the morpholinyl protons and downfield singlet at d 13.79 ppm assigned for the NH proton in addition to the presence of aromatic protons. A molecular ion peak was observed in the mass spectrum of compound 7b at m/z = 391(100 %) corresponding to the molecular formula C22H18FN3OS, Chart II. The formation of 7 is assumed to proceed via the addition of active methylene of cyanothioacetamide to the double bond of 2 to give Michael intermediate 5, which then undergoes heterocyclization, dehydration [30] and dehydrogenation to yield 7, (Scheme 3). In a similar manner, cyclocondensation of a,b-unsaturated compounds 2a,b with cyanoacetamide in refluxing ethanol in the presence of sodium ethoxide afforded the corresponding 2-oxo-1,2-dihydropyridine-3-carbonitriles 8a,b. The structures of 8a,b were established on the basis of their elemental analysis and spectral data. The infrared spectra of compounds 8a, b indicated the presence of the NH, CH-aliph., C≡N and C=O groups. Also, the 1HNMR spectrum (DMSO-d6) of compound 8a revealed two triplets at δ 3.31; 3.74 ppm assigned to the morpholinyl protons, a multiplet at δ 6.93-8.08 ppm assigned aromatic protons in addition to the presence of downfield singlet at δ 12.6 ppm assigned to the NH proton.
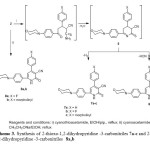 |
Scheme3: Synthesis of 2-thioxo-1,2-dihydropyridine -3-carbonitriles 7a-c and 2-oxo-1,2-dihydropyridine -3-carbonitriles 8a,b Click here to View scheme |
 |
Chart 2 Click here to View chart |
The reaction of compound 2a with ethyl cyanoacetate was also investigated. Reaction of compound 2a with ethyl cyanoacetate in refluxing ethanol in the presence of catalytic amounts of piperidine afforded ethyl 6-(4-morpholinophenyl)-2-oxo-4-phenyl-1,2-dihydro-pyridine-3-carboxylate 12 , (Scheme 4) . The other possible structure 11 was readily excluded on the basis of elemental analysis and spectral data. The infrared spectrum of the reaction product revealed the lake of absorption band corresponding to a nitrile function and showed the characteristic absorption bands at 3439, 2961, 2845 cm-1 for the NH and CH-aliph and two carbonyl groups at 1742, 1648 cm-1. The 1HNMR spectrum (DMSO-d6) of the reaction product revealed three triplet at δ 1.08, 3.34, 3.74 ppm assigned to the methyl and morphonyl protons, a quartet at δ 4.10 ppm assigned to the methylene protons, a multiplet at δ 6.95-8.08 ppm assigned to the aromatic protons and downfield signal assigned for the NH proton The mass spectrum of compound 12 showed a molecular ion peak at m/z = 404 (1.33%) corresponding to the molecular formula C24H24N2O4 with base peak m/z = 190. The formation of 12 is assumed to proceed via addition of the active methylene group of ethyl cyanoacetate to the double bond of 2a to give Michael adduct 9 that readily cyclize to generate pyranimine 10, which undergo a Dimroth rearrangement [31] and dehydrogenation to give pyridone derivative 12, (Scheme 4).
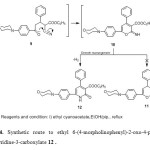 |
Scheme4: Synthetic route to ethyl 6-(4-morpholinophenyl)-2-oxo-4-phenyl-1,2-dihydropyridine-3-carboxylate 12 . Click here to View scheme |
The reaction of cyanoacetohydrazone derivative 3 with cinnamonitrile derivatives was studied. Thus, the reaction of cyanoacetohydrazone derivative 3 with cinnamonitriles in ethanol containing catalytic amount of piperidine gave the 6-amino-4-aryl-2-oxo-1,2-dihydropyridine-3,5-dicarbonitrile 14a,b, (Scheme 5). The structure of pyridine derivatives 14a,b were established on the basis of their elemental analysis and spectral data. The infrared spectra of compounds 14a,b indicated the characteristic absorption bands for the NH2, CH-aliph., C≡N and C=O groups. The 1HNMR spectrum (DMSO-d6) of compound 14a displayed a singlet at δ 2.45 ppm assigned to the methyl protons, two triplets δ 3.26, 3.74 ppm assigned to the morphonyl protons, a multiplet at 6.76-7.96 ppm assigned to the aromatic protons and downfield singlet at d 8.56 ppm assigned to the amino protons. The mass spectrum of compound 14a showed a molecular ion peak at m/z = 438 (1%) corresponding to the molecular formula C25H22N6O2. The formation of 14 is assumed to proceed via Michael addition of the methylene group in 3 to the activated double bond in cinnamonitrile to give Michael adduct 13 which cyclized and oxidized [32] under the reaction conditions to yield 14, (Scheme 5).
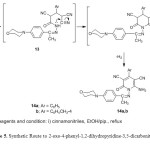 |
Scheme5: Synthetic Route to 2-oxo-4-phenyl-1,2-dihydropyridine-3,5-dicarbonitriles 14a,b. Click here to View scheme |
Cytotoxic Activity
In attempts to find new anticancer compounds, we reported synthesis of various pyridones and pyridinethione derivatives. In vitro cytotoxicity assays of these compounds were performed on human lung cancer cell line (A 549) by colorimetric method [33]. The results expressed as IC50 (inhibitory concentration of 50 are listed in Table 5). The results were compared with standard reference drug Doxorubicin as a reference drug versus lung carcinoma cell line (A 549). Fig. 1 represented cytotoxic activities of compounds on a single cell line (A 549). Comparison of the activity of the investigated series indicated that:
- The antitumor activity of these compounds obeyed this order 8a (IC50 = 0.83 μg/ml) > 7b (IC50 = 0.87 μg/ml) > 7a (IC50 = 1.25 μg/ml) > 7c (IC50 = 1.38 μg/ml) > 2a (IC50 = 4.63 μg/ml) > 3 (IC50 = 6.95 μg/ml) > 12 (IC50 = 8.42 μg/ml) > 8b (IC50 = 8.88 μg/ml) > 2c (IC50 = 9.01 μg/ml) 4 (IC50 = 9.08 μg/ml) > 2b (IC50 = 10.50μg/ml).
- Compounds 8a and 7b which have para fluoro substituent on phenyl ring attached to pyridone moiety exerted better activity with IC50 value in the range of 0.83 (Fig. 2) and 0.87 μg/ml (Fig.3) respectively versus lung carcinoma cell line (A549).
- Compounds 2b, 4 and 2c have lower antitumor activity among the synthesized compounds with IC50 of 10.5, 9.08 and 9.01 μg/ml respectively versus (A549).
- Compounds 8a and 7b have more antitumor activity than Doxrubcin as standard drug. This means that compounds 8a and 7b possess the most potent inhibitory effect against the human lung carcinoma cell line (A549).
- It is notable that 7a and 7c have comparable activity to that Doxrubcin standard drug.
- As shown in Table 1, most of these compounds low, moderate and highly potent activity against lung carcinoma cell line (A549), indicating that they could be serving as promising drugs for cancer.
- Also, more detailed and advanced studies are needed to fully understand the detailed molecular mechanism of cytotoxicity
- Compounds 2a and 3 have moderate antitumor activity compared to Doxrubcin standard drug.
- The electron withdrawing fluorine substitution on phenyl ring retained the cytotoxicity of parent ring.
Table 1. Cytotoxicity assay in vitro for the synthesized compounds.
|
Compound number |
IC50a |
|
2a |
4.63 |
|
2b |
10.5 |
|
2c |
9.01 |
|
3 |
6.95 |
|
4 |
9.08 |
|
7b |
0.87 |
|
7a |
1.25 |
|
7c |
1.38 |
|
8a |
0.83 |
|
8b |
8.88 |
|
12 |
8.42 |
|
Doxrubcin |
0.93 |
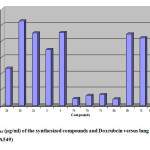 |
Figure1: IC50 (μg/ml) of the synthesized compounds and Doxrubcin versus lung carcinoma cell line (A549) Click here to View figure |
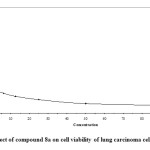 |
Figure2: Effect of compound 8a on cell viability of lung carcinoma cell line (A549). Click here to View figure |
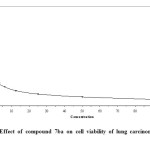 |
Figure3: Effect of compound 7ba on cell viability of lung carcinoma cell line (A549) Click here to View figure |
Conclusions
A series of novel pyridine derivatives were designed and synthesized. All the prepared compounds were characterized by spectral data (1H-NMR and IR) and elemental analysis. The antitumor activity of the newly synthesized products were investigated, showing that compound exhibited low, moderate to highly potent activity compared to Doxorubicin standard drug.
Experimental
Chemistry
Melting points were determined on a Gallenkamp apparatus and uncorrected. The purity of the compounds was checked by TLC. IR spectra were recorded in a Pye-Unicam SP300 instrument in potassium bromide discs. 1H NMR spectra was recorded in a Varian Mercury VXR-300 spectrometer (300 MHz for 1HNMR) in DMSO-d6 and the chemical shifts were related to that of the solvent. Mass spectra were recorded in a GCMS-QP 1000 EX Shimadzu Spectrometer, the ionizing voltage was 70 eV. Elemental analyses were carried out in the Microanalytical Laboratory of Cairo University, Giza, Egypt. Antitumor activity was performed in RegionalCenter for Mycology and Biotechnology, Al-AzharUniversity, Cairo, Egypt.
General procedure for the preparation of chalcones 2a-c: A mixture of ethanone 1 (0.01 mol), aldehyde(0.01 mole) and 2 N sodium hydroxide (5 mL) in ethanol (30 mL) was stirred at room temperature for 1h. The precipitated solid was filtered, wash with water, dried and recrystallized from the proper solvent.
(E)-1-(4-morpholinophenyl)-3-phenylprop-2-en-1-one (2a)
This compound was obtained in 82% yield as yellow crystals (from dioxan), m.p.: 176-177°C, IR (KBr, cm-1): 2961, 2846 (CH-aliph), 1647(C=O) and 1607(C=C). 1HNMR (300 MHz, DMSO-d6): d 3.32, 3.71(2t, 8H, morphonyl-H), 7.02(d, 1H, J=15.5 Hz), 7.43-7.85(m, 9H, Ar-H) and 8.09 (d, 1H, J=15.5 Hz). Anal. Calcd. For C19H19NO2: C, 77.79; H, 6.53; N, 4.77. Found: C, 77.20; H, 6.70; N, 4.10.
(E)-3-(4-fluorophenyl)-1-(4-morpholinophenyl)prop-2-en-1-one (2b)
This compound was obtained in 87% yield as yellow crystals (from Dioxan), m.p.:187-188°C; IR (KBr, cm-1): 2972, 2846(CH-aliph), 1648(C=O) and 1609 (C=C). 1HNMR (300 MHz, DMSO-d6): d3.31, 3.73(2t, 8H, morphonyl-H), 7.01(d, 1H, J=15.6 Hz), 7.25-7.96 (m, 8H, Ar-H) and 8.08(d, 1H, J=15.6 Hz). Anal. Calcd. For C19H18FNO2: C, 73.29; H, 5.83; N, 4.50. Found: C, 72.80; H, 5.15; N, 4.00.
(E)-1,3-bis (4-morpholinophenyl) prop-2-en-1-one (2c)
This compound was obtained in 84% yield as orange crystals (from Dioxan), m.p.:151-152°C; IR (KBr, cm-1): 2963, 2846(CH-aliph), 1648(C=O) and 1594(C=C). 1HNMR (300 MHz, DMSO-d6):d 3.26, 3.71(2t, 16H, morphonyl-H), 6.96 (d, 1H, J=15.8 Hz), 7.34-7.70 (m, 8H, Ar-H) and 8.18 (d, 1H, J=15.8 Hz). Anal. Calcd. For C23H26N2O3: C, 72.99; H, 6.92; N, 7.40. Found: C, 72.60; H, 6.40; N, 7.60;
2-cyano-N’-(1-(4-morpholinophenyl)ethylidene)acetohydrazide (3)
A mixture of cyanoacetic acid hydrazide (0.01 mol) and ethanone 1 (0.01 mol) in absolute ethanol (20 mL) was refluxed for 1 h. After cooling the separated crystalline products was filtered, wash with ethanol, dried and recrystallized from the proper solvent. This compound was obtained in 88% yield as colorless crystals from ethanol, m.p.: 100-101°C; IR (KBr, cm-1): 3347 (NH), 2977, 2842 (CH-aliph), 2260 (C≡N), 1660 (C=O) and 1601(C=N). 1HNMR (300 MHz, DMSO-d6): d 2.45 (s, 3H, CH3), 3.28, 3.71 (2t, 8H, morphonyl-H); 4.35 (s, 2H, CH2), 6.96, 7.80 (2d, 4H, Ar-H) and 8.27 (s, 1H.NH, canceled with D2O). Anal. Calcd. For C15H18N4O2: C, 62.92; H, 6.34; N, 19.57. Found: C, 63.00; H, 6.10; N, 19.20.
(E)-3-(dimethylamino)-1-(4-morpholinophenyl) prop-2-en-1-one (4)
To a solution of ethanone 1 (0.01mol) in dry xylene (10 mL), dimethyl formamide-dimethylacetal (0.01mol) was added. The reaction mixture was heated under reflux for 4h. The solvent was removed by evaporation under reduced pressure and the remainder was left to cool. The solid product so formed was collected by filtration, washed with petroleum ether (b.p 40-60°C), and the crude product recrystallized from proper solvent. This compound was obtained in 72% yield as yellow crystals from ethanol, m.p.:198-199°C; IR (KBr, cm-1): 2968, 2817 (CH-aliph), 1636(C=O) and 1601(C=C). 1HNMR (300 MHz, DMSO-d6): d 3.19 (br, 6H, 2CH3), 3.20, 3.75 (2t, 8H, morphonyl-H); 5.76 (d, 1H, J=12.5 Hz), 6.91, 7.79 (2d, 4H, Ar-H) and 7.60 ( d, 1H, J=12.5Hz) . Anal. Calcd. For C15H20N2O2: C, 69.20; H, 7.74; N, 10.76. Found: C, 68.90; H, 7.50; N, 10.30.
General procedure for the preparation of 2-thioxo- 1, 2-dihydropyridine -3-carbonitriles7a-c.
A mixture of compound 2 (0.01 mol), cyanothioacetamide (0.01 mole) and piperdine (0.01 mol) in 20 mL of ethanol was refluxed for 2 h, then allowed to cool, and poured into cold water and acidified with HCl. The solid product was collected and recrystallized from the proper solvent.
6- (4- Morpholinophenyl)-4- phenyl -2- thioxo- 1, 2-dihydropyridine -3-carbonitrile (7a)
This compound was obtained in 84% yield as orange crystals (from ethanol), m.p.: 115-116°C, IR (KBr, cm-1): 3380 (NH) 2985, 2854 (CH-aliph), 2220 (C≡N) and 1227 (C=S). 1HNMR (300 MHz, DMSO-d6): d 3.16, 3.74 (2t, 8H, morphonyl-H) 6.94-7.86 (m, 10H, Ar-H), and 13.79 (s, 1H, NH, cancelled with D2O). Anal. Calcd. For C22H19N3OS: C, 70.75; H, 5.13; N, 11.25. Found: C, 70.30; H, 4.70; N, 10.80.
4-(4-Fluorophenyl)-6-(4-morpholinophenyl)-2-thioxo-1,2-dihydropyridi-n-e-3-carbonitrile (7b)
This compound was obtained in 75% yield as red crystals (from ethanol), m.p.180-181°C; IR (KBr, cm-1): 3335 (NH). 2956, 2854(CH-aliph), 2217 (C=N) and 1227 (C=S). 1HNMR (300 MHz, DMSO-d6): 3.23, 3.75 (2t, 8H, morphonyl-H), 6.84- 8.07 (m, 9H, Ar-H) and 13.78 (s, 1H, NH, cancelled with D2O). Anal. Calcd. For C22H18FN3OS: C, 67.50; H, 4.63; N, 10.73. Found: C, 67.10; H, 4.15; N, 10.90.
4,6-bis(4-morpholinophenyl)-2-thioxo-1,2-dihydropyridine-3-carbonitrile (7c)
This compound was obtained in 73% yield as orange crystals (from ethanol), m.p.:118-119°C; IR (KBr, cm-1): 3421(NH). 2937, 2853 (CH-aliph), 2217 (C≡N) and 1230 (C=S). 1HNMR (300 MHz, DMSO-d6): 3.27, 3.75 (2t, 16H, morphonyl-H), 6.91-8.27(m, 9H, Ar-H) and 13.95 (s, 1H, NH, cancelled with D2O). Anal. Calcd. For C26H26N4O2S: C, 68.10; H, 5.71; N, 12.22. Found: C, 67.70; H, 5.60; N, 11.90.
General procedure for the preparation of 2-oxo – 1, 2-dihydropyridine -3-carbonitriles 8a-c.
A mixture of compound 2 (0.01 mol) and cyanoacetamide (0.01 mole) was refluxed in sodium ethoxide (0.1 Na in 25 mL absolute ethanol) for 3 h, then allowed to cool and poured into cold water (50 mL) and acidified with HCl. The solid product was collected and recrystallized from the proper solvent.
4-(4-fluorophenyl)-6-(4-morpholinophenyl)-2-oxo-1,2-dihydropyridine-3-carbonitrile (8a)
This compound was obtained in 67% yield as yellow crystals (from ethanol), m.p.:130-131 °C, IR (KBr, cm-1): 3400 (NH) 2964, 2855 (CH-aliph), 2216 (C≡N) and 1651 (C=O). 1HNMR (300 MHz, DMSO-d6): δ 3.27, 3.74 (2t, 8H, morphonyl-H), 6.93-8.08 (m, 9H, Ar-H), and 12.6 (s, 1H, NH, cancelled with D2O). Anal. Calcd. For C22H18FN3O2: C, 70.39; H, 4.83; N, 11.19. Found: C, 70.00; H, 4.55; N, 10.80.
4, 6-bis (4-morpholinophenyl)-2-oxo-1, 2-dihydropyridine-3-carbonitrile (8b)
This compound was obtained in 65% yield as yellow crystals (from ethanol), m.p.:120-121 °C, IR (KBr, cm-1): 3420 (NH) 2950, 2853 (CH-aliph), 2215 (C≡N) and 1644 (C=O). 1HNMR (300 MHz, DMSO-d6): δ 3.22, 3.76 (2t, 16H, morphonyl-H), 6.79-8.00 (m, 9H, Ar-H), and 12.2 (s, 1H, NH, cancelled with D2O). Anal. Calcd. For C26H26N4O3: C, 70.57; H, 5.92; N, 12.66. Found: C, 70.15; H, 5.70; N, 12.10.
Ethyl 6-(4-morpholinophenyl)-2-oxo-4-phenyl-1,2-dihydropyridine-3-carboxylate (12)
To a mixture of compound 2a (0.01 mol) and ethyl cyanoacetate (0.01 mol) in ethanol (25 mL), a few drops of piperidine was added. The reaction mixture was refluxed of 4 h, then allowed to cool, and poured into cold water (50 mL) and acidified with HCl. The solid product was collected and recrystallized from the proper solvent.This compound was obtained in 62% yield as yellow crystals (from ethanol), m.p.: 122-123 °C ; IR (KBr, cm-1 ): 3439 (NH) 2961, 2845 (CH-aliph), 1742 (C=O; ester) 1666 (C=O ; pyridone) 1HNMR (300 MHz, DMSO-d6): 1.08 (t, 3H, CH3) d 3.28, 3.74 (2t, 8H, morphonyl-H), 4.20 (q, 2H, CH2 ) 6.95- 8.08 (m, 9H, Ar-H) , and 12.4 (s, 1H, NH, cancelled with D2O). Anal. Calcd. For C24H24N2O4: C, 71.27; H, 5.98; N, 6.93. Found: C, 70.80; H, 5.40; N, 7.10.
General procedure for the preparation of 2-oxo-4-phenyl-1,2-dihydropyridine-3,5-dicarbonitriles 14a, b
To a solution of 3 (0.01mol) in absolute ethanol (20 mL) was added the appropriate cinnamonitrile (0.01 mol) and two drops of piperidine. The reaction mixture was refluxed for 3h. The mixture was cooled and the separated crystalline product was filtered, washes with ethanol, dried and recrystallized from the proper solvent.
6-Amino-1-((1-(4-morpholinophenyl)ethylidene) amino)-2-oxo-4-phenyl-1,2-dihydropyridine-3,5-dicarbonitrile (14a)
This compound was obtained in 65% yield as orange crystals (from ethanol), m.p.: 75-76 °C; IR (KBr, cm-1): 3354, 3146 (NH2), 2980, 2850 (CH-aliph), 2218 (C≡N), 1636 (C=O) and 1595 (C=N). 1HNMR (300 MHz, DMSO-d6): 2.4 (s, 3H, CH3) δ 3.26, 3.74 (2t, 8H, morphonyl-H), 6.76-7.96 (m, 9H, Ar-H), and 8.56 (s, 2H, NH2, cancelled with D2O). Anal. Calcd. For C25H22N6O2: C, 68.48; H, 5.06; N, 19.17. Found: C, 68.00; H, 4.75; N, 18.80.
6-amino-1-((1-(4-morpholinophenyl)ethylidene)amino)-2-oxo-4-(p-tolyl)-1, 2-dihydropyridine-3, 5-dicarbonitrile (14b).
This compound was obtained in 63% yield as yellow crystals (from ethanol), m.p.: 116-117 °C; IR (KBr, cm-1): 3347, 3200 (NH2), 2924, 2840 (CH-aliph), 2225 (C≡N), 1644 (C=O) and 1605 (C=N). 1HNMR (300 MHz, DMSO-d6): 2.37, 2.41 (2s, 6H, 2CH3) δ 3.30, 3.73 (2t, 8H, morphonyl-H), 7.42 -7.85 (2d, 8H, Ar-H), and 8.47 (s, 2H, NH2, cancelled with D2O). Anal. Calcd. For C26H24N6O2: C, 69.01; H, 5.35; N, 18.57. Found: C, 68.70; H, 5.50; N, 18.80.
Antitumor Activity
Compounds 2a-c,3,4,7a-c, 8a,b, and 12, were tested for their cytotoxicity in vitro, in comparison with doxorubicin (DXR) as a reference drug against Lung carcinoma cells (A 549). Human lung carcinoma (A 549) cell line was obtained from the American Type Culture Collection (ATCC, Rockville, MD). The cells were grown on RPMI-1640 medium supplemented with 10% inactivated fetal calf serum and 50µg/ml gentamycin. The cells were maintained at 37ºC in a humidified atmosphere with 5% CO2 and were subcultured two to three times a week. For antitumor assays, the tumor cell lines were suspended in medium at concentration 5×104 cell/well, and then incubated for 24 hr. The tested compounds were then added into 96-well plates (six replicates) to achieve eight concentrations for each compound. Six vehicle controls with media or 0.5 % DMSO were run for each 96 well plate as a control. After incubating for 24 h, the numbers of viable cells were determined by the MTT test [34]. Briefly, the media was removed from the 96 well plates and replaced with 100 µl of fresh culture RPMI 1640 medium without phenol red then 10 µl of the 12 mM MTT stock solution (5 mg of MTT in 1 mL of PBS) to each well including the untreated controls. The 96 well plates were then incubated at 37°C and 5% CO2 for 4 hours. An 85 µl aliquot of the media was removed from the wells, and 50 µl of DMSO was added to each well and mixed thoroughly with the pipette and incubated at 37°C for 10 min. Then, the optical density was measured at 590 nm with the microplate reader (TECAN, Inc, USA) to determine the number of viable cells and the percentage of viability was calculated as [1-(ODt/ODc)]x100% where ODt is the mean optical density of wells treated with the tested sample and ODc is the mean optical density of untreated cells. The 50% inhibitory concentration (IC50), the concentration required to cause toxic effects in 50% of intact cells, was estimated from graphic plots of the dose response curve for each concentration using Graphpad Prism software (San Diego, CA. USA).
Acknowledgments
The authors express their sincere thanks to the Northern Border University for financial support of the project number (435-810-8).
References
- Kiuru, P.; Yli-Kauhaluoma, J. Pyridine and Its Derivatives. In Heterocycles in natural product synthesis; Majumdar, K.; Chattopadhyay, S. K., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; 267–297.
- Baumann, M.; Baxendale, I. R. Beilstein J. Org. Chem. 2013, 9, 2265–2319.
- Chaubey A.; Pandeya, S. N. Asian J. Pharm. Clin. Res, 2011, 4(4), 5-8.
- Liu, C.; Luo, J.; Xu, L.; Huo, Z. Arkivoc, 2013, i , 154-174.
- Shishoo, C. J.; Devani, M. B.; Bhadti, V. S.; Ananthan, S.; Ullas, G. V. Tetrahedron Lett. 1983, 24, 4611-4612.
- Ghosh, P. S.; Manna, K.; Banik, U.; Das, M.; Sarkar, P. Int. J. Pharm. Pharm. Sci.2014, 6(4), 39-42.
- Aly, A. A. Phosphorus Sulfur Silicon Relat. Elem. 2006, 181, 2395-2409.
- Barili, P. L.; Biagi, G.; Livi, O.; Mucciand, L.; Scartoni, V. J. Heterocycl. Chem. 1987, 24, 997-1001.
- Kumar, N.; Singh, G.; Yadav, A. K. Heteroat. Chem. 2001, 12, 52–56.
- Khatoon, S.; Yadav, A. K. Phosphorus Sulfur Silicon Relat. Elem. 2004, 179, 345-352.
- Ravikanth, S.; Venkat Reddy, G.; Maitraie, D.; Rama Rao, V.; Shanthan Rao, P.; Narsaiah, B. Synth. Commun. 2004, 34, 4463-4469.
- Fleming, F. F.; Yao, L.; Ravikumar, P. C.; Funk, L.; Shook, B. C. J. Med. Chem. 2010, 53, 7902–7917.
- Mirkovi, J. M.; Mijin, D. Z.; Petrovic, S. D. Hem. Ind. 2013, 67 (1), 17–25.
- Cheney, I. W.; Yan, S.; Appleby, T.; Walker, H.; Vo, T.; Yao, N.; Hamatake, R.; Hong, Z.; Wu, J. Z. Bioorg. Med. Chem. Lett. 2007, 17, 1679-1683.
- Lebedyeva, I. O.; Dotsenko, V. V.; Turovtsev, V. V.; Krivokolysko, S. G. ; Povstyanoy, V. M.; Povstyanoy M. V. Tetrahedron 2012, 68 , 9729-9737.
- Singh, P.; Anand, A.; Kumar, V. Eur. J. Med. Chem. 2014, 85, 758-777.
- Zulkepli, N. A.; Rou, K. V. K.; Sulaiman, W. N. H. W.; Salhin, A.; Saad, B.; Seeni, A. Asian Pac. J. Cancer Prev. 2011, 12, 259–263.
- El-Gaby, M. S. A.; El-Hag Ali G. A. M.; El-Maghraby, A. A.;Abd El-Rahman, M. T.; Helal, M. H. M. Eur. J. Med. Chem, 2009,44 (10), 4148-4152.
- Helal, M. H. M.; Salem, M. A.; El-Gaby, M. S. A.; Aljahdali, M. Eur. J. Med. Chem. 2013, 65, 517- 526.
- El-Hag Ali, G. A.; Abd El-Rahman, M. M. T.; Helal M. H. M.; El-Gaby, M. S. A. Phosphorus Sulfur Silicon Relat. Elem. 2008, 183(12), 3023-3036.
- Helal, M. H.; Abbas S. Y.; Salem M. A.; Farag A. A.; Ammar Y. A. Med. Chem. Res. 2013, 22, 5598-5609.
- Helal, M. H. M.; Ahmed, N. S.; Elwessaly, M.; Ammar, Y. A. Arch. Pharm. Chem. Life Sci. 2014, 347(2), 123–133.
- Helal, M. H.; El-Awdan, S. A.; Salem, M.A.; Abd-elaziz, T.A.; Moahamed, Y.A.; El-Sherif, A. A.; Mohamed, G.A.M. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2015, 135, 764–773.
- Moustafa, A. G.; Mohamed, H. H. Eur. J. Chem. 2015, 6(1), 84-88.
- Mohamed,H. H.;Gameel, A. M.; Ahmed, A. A.; Yousry A. A. J. Chemical Research 2010, 34(8), 465-469.
- Bader, H.; Hansen, A. R.; McCarty, F. J. J. Org. Chem. 1966, 31, 2319-2321.
- Ning, Y. Interpretation of organic spectra, 1st ed., John Wiley & Sones 2011, 393.
- Shaaban, M. R.; Salah, T. S.; Osman, F. H.; Farag, A. M. J. Heterocycl. chem. 2007, 44, 177-181.
- Chebanov, V. A.; Desenko, S. M.; Gurley, T. W. Azaheterocycles based ona,b-unsaturated carbonyls, Springer-Verlag Berline Heidelberg, 2008.
- Krauze, A.; Germane, S.; Eberlins O.; Sturms, I.; Klusa, V.; Duburs. G. Eur. J. Med. Chem. 1999, 34, 301.
- Gorobets, N. Y.; Sedash, Y. V.; Shishkina, S. V.; Shishkina, O. V.; Yermolayev, S. A.; Desenko, S. M. Arkivoc 2009,Xiii, 23.
- Soto, J. L.; Seoane, C.; Zamorano, P.; Cuadrado, F. J. Synthesis 1981, 7, 529.
- Elaasser, M. M.; Abdel-Aziz, M. M.; El-Kassas, R. A. J. Microbiol Biotech Res. 2011, 1:5-17.
- Harper, D. R.; Determination of viral infectivity. In: Methods in molecular medicine, Vol. 24: Antiviral methods and protocols. (Edited by Kinchington, D. and Schinazi, R. F.) Humana Press Inc., Totowa, N J., 2000, 119-124.

This work is licensed under a Creative Commons Attribution 4.0 International License.









