Copper (I) iodide nanoparticles on polyaniline as a green, recoverable and reusable catalyst for multicomponent click synthesis of 1,4-disubstituted-1H-1,2,3-triazoles
Shervin Saadat1*, Simin Nazari2, Mozhgan Afshari3, Maryam Shahabi4, Mosadegh Keshavarz4*
1Faculty of Science, Ahvaz Branch, Islamic Azad University, Ahvaz, Iran.
2Department of Chemistry, Sousangerd Branch, Islamic Azad University, Sousangerd 44181-6189, Iran.
3Department of Chemistry, Shoushtar Branch, Islamic Azad University, Shoushtar, Iran.
4Department of Gas and Petroleum, Yasouj University, Gachsaran, Iran.
e-mail: chem.mosadegh@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/310248
Article Received on :
Article Accepted on :
Article Published : 10 Jun 2015
A one-pot procedure for the synthesis of 1,4-disubstituted-1H-1,2,3-triazole derivatives via the three component coupling reaction between terminal alkynes, benzyl halides/α-halo ketones and sodium azide in the presences of CuI nanoparticles supported onto polyaniline (Nano CuI/PANI) catalyst in water has been developed. This heterogeneous catalyst showed high catalytic activity and 1,4-regioselectivity for click cyclization in water as a “green” solvent and good to excellent yields were obtained in all cases over repeated five runs.
KEYWORDS:Nanoparticles; Click chemistry; Heterogeneous catalyst; Benzyl halide; α-halo ketones; Triazole
Download this article as:| Copy the following to cite this article: Saadat S, Nazari S, Afshari M, Shahabi M, Keshavarz M. Copper (I) iodide nanoparticles on polyaniline as a green, recoverable and reusable catalyst for multicomponent click synthesis of 1,4-disubstituted-1H-1,2,3-triazoles. Orient J Chem 2015;31(2). |
| Copy the following to cite this URL: Saadat S, Nazari S, Afshari M, Shahabi M, Keshavarz M. Copper (I) iodide nanoparticles on polyaniline as a green, recoverable and reusable catalyst for multicomponent click synthesis of 1,4-disubstituted-1H-1,2,3-triazoles. Available from: http://www.orientjchem.org/?p=9186 |
Introduction
1,2,3-Triazoles are an important class of heterocyclic compounds thanks to their wide range of applications. 1 The most fashionable method for the production of the 1,2,3-triazole framework is the 1,3-dipolar Huisgen cycloaddition reaction of azides with alkynes .2 The Cu(I)-catalyzed azide-alkyne
cycloaddition (CuAAC),2,3 one of the most consistent “click reactions”,4 has enabled the convenient and efficient preparation of 1,4-disubstituted-1,2,3-triazoles, from a wide range of substrates with excellent selectivity, which cannot be prepared by means of traditional Huisgen uncatalyzed thermal approaches.5
Copper salts are toxic compounds. Despite of toxicity, copper (I) salts suffer from their thermodynamic instability and the formation of undesired alkyne-alkyne coupling products which is sometimes observed in its presence.6 These homogenous catalysts are generally non-recyclable, have low regioselectivity and long reaction times. Nevertheless, Polymer-supported reagents are more and more being used in the synthesis and purification of many organic reactions .7,8 They possess advantages such as reaction monitoring as well as increased safety, in particular when the non-supported reagents are toxic or dangerous as they can be easily removed from reaction media and recycled.9,10
Nitrogen or phosphorus-based ligands have been shown to protect the metal center from oxidation and disproportionation, while enhancing its catalytic activity.11 To improve the recovery and reuse, copper species have been immobilized onto various supports.12-15
In continue to our previous studies in the field of click chemistry, 12-19 herein we wish to report a new and reused catalyst system based on CuI nanoparticles on polyaniline (PANI@CuI-NPs) for click cyclization.
Results and discussion
Polyaniline (PANI) was chosen as a support material due to its unique characteristics such as superficial preparative protocols from inexpensive starting material (aniline), high environmental stability and non-solubility in most of the organic solvents. Some types of PANI-supported metal catalysts have been synthesized and applied in modern organic synthesis. 20,21
The copper iodide nanoparticles immobilized on polyaniline (PANI@CuI-NPs) was readily prepared in a one-step procedure. PANI was refluxed with CuI under an N2 atmosphere in EtOH to afford the polymer-supported CuI nanoparticles catalyst. The immobilization of copper(I) salt nanoparticles on high-surface-area supports allows a higher stability and dispersity of the particles as well as a further exploitation of the special activity and recycling properties of the catalyst. With this regard, we reported the similar procedure for the preparation heterogeneous poly(4-vinylpyridine) supported CuI nanoparticles (P4VPy-CuI) as catalyst for click synthesis of 1,4-disubstituted triazoles.12,15,18
The SEM image of PANI@CuI-NPs indicated that CuI nanoparticles were homogeneously immobilized on PANI surface (Figure 1). The SEM image shows the detailed morphologies of the CuI nanoparticles deposited on the surface of PANI which are highly disperse without any aggregation and approximately uniform in size distribution. The size and microstructure of CuI nanoparticles were further examined with field emission scanning electron microscopy (FE-SEM). The morphology of the CuI nanoparticles can be clearly observed. The size of each SnS nanoparticles is in the range of 20-60 nm.
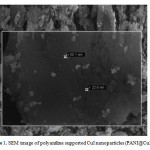 |
Figure1: SEM image of polyaniline supported CuI nanoparticles (PANI@CuI-NPs) Click here to View figure |
The sharp peaks of copper were observed in the XRD patterns of copper iodide on PANI which indicated the crystalline nature of the products and confirmed the presence of copper iodide on PANI surface. The copper content of PANI@CuI-NPs was measured by ICP-AES and found to be 0.94 mmol g-1.
In order to exploit a method for the preparation of new 1,2,3-triazole derivatives, the reaction of phenacyl bromide, phenylacetylene and sodium azide in the presence of PANI@CuI-NPs through a 1,3-dipolar Huisgen cycloaddition reaction was chosen as a model and its behavior was studied under a variety of conditions. Under optimization studies, various solvents were examined and it was found that the solvent plays a significant role in terms of reaction rate and isolated yield (Table 1).
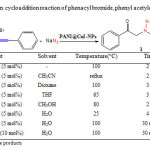 |
Table1: 1,3-Dipolar Huisgen cycloaddition reaction of phenacyl bromide, phenyl acetylene and sodium azide under various conditions. Click here to View table |
Water evidently stands out as the solvent of preference with its fast reaction rate, high yield, selectivity, cheapness, “green” solvent nature and environmental acceptability. An increase in the amount of PANI@CuI-NPs did not affect the efficiency of the reaction (Table 1, entry 7 versus 8). Structural assignments of compound 1 and other triazoles were confirmed by the appearance of a singlet in the region of 7.5-8.5 ppm in 1H-NMR spectra and approved the regioselective synthesis of 1,4-disubstituted triazole regioisomers.16
Based on the optimal reaction condition, various alkyne were allowed to react with azide compounds in the presence of PANI@CuI-NPs (5.0 mol%) in water (Scheme 1). Phenylacetylene and electron-rich aromatic alkynes gave excellent results with benzyl azide. However electron-deficient aromatic alkyne toke longer times to afford 1, 2, 3-triazole products under the same reaction conditions. The reaction only gave good yield even that the reaction time was prolonged to 2 h. The electronic effects on the resulting yields and conversion times of the reactions are different Heterocyclic alkyne, such as 2-thienylacetylene, also gave high yields in short time. Aliphatic alkyne required longer reaction time to give high yields in comparison with electron-rich aromatic alkyne, but the reactions were finished within 2 h (Table 2, entries 7-9).
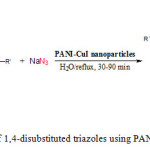 |
Scheme1: Synthesis of 1,4-disubstituted triazoles using PANI@CuI-NPs as catalyst |
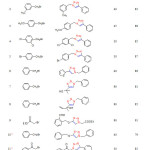 |
Table2: The reaction of α-halo ketones and benzyl halide (1 mmol), terminal alkyne (1 mmol) and sodium azide (1.1 mmol) catalyzed by PANI@CuI-NPs (5 mol %) in water at 100 °C Click here to View table |
To evaluate the efficiency and superiority of PANI@CuI-NPs catalyst, we performed some reactions using PANI@CuI-NPs and unsupported bulk CuI at the same reaction conditions. The reaction of benzyl bromide with phenyl acetylene was completed and found 89℅ formation of the desired 1,2,3-triazole as only product after 30 min using PANI@CuI-NPs (5%) versus 33℅ in the case of unsupported CuI. This reaction was not completed using unsupported CuI (10%) even after 2 h. It is worth to note that trace amount of alkyne-alkyne coupling products were detected in the presence of unsupported CuI (Table 3).
Table 3. Activity comparison of PANI@CuI-NPs and unsupported CuI in click cyclization reaction
|
Entry |
Benzyl halide/ α-halo ketones |
Catalyst |
Alkyne-Alkyne byproduct |
Yield(%)a |
|
1 |
Benzyl bromide |
PANI@CuI-NPs (5%) |
– |
89 b |
|
2 |
Benzyl bromide |
CuI(5%) |
Trace |
33 b |
|
3 |
Phenacyl bromide |
PANI@CuI-NPs (5%) |
– |
90 b |
|
4 |
Phenacyl bromide |
CuI(5%) |
Trace |
50 b |
|
5 |
Benzyl bromide |
CuI(10%) |
Trace |
70 c |
|
6 |
Phenacyl bromide |
CuI(10%) |
Trace |
65 c |
a Yield refer to isolated and pure product
bReaction conditions: benzyl halide(1 mmol), sodium azide(1.1 mmol), water(5 ml), 100 °C, 30 min
cReaction was not completed after 240 min
Here most probably, PANI has dual roles as a support as well as a macro ligand which facilitate the cycloaddition reaction as reported by using other nitrogen containing ligands.22,23 Immobilization of copper iodide on PANI protects it against oxidation and enhances its catalytic activity in comparison with unsupported copper iodide. PANI has effective influence in step (2) of click cyclization mechanism. PANI makes hydrogen bond with the acidic hydrogen of terminal alkyne which results in the faster rate of the deprotonation step (Figure 2).
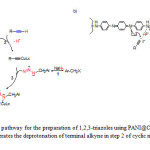 |
Figure2: a) Mechanistic pathway for the preparation of 1,2,3-triazoles using PANI@CuI-NPs as catalyst in water, b) PANI@CuI-NPs accelerates the deprotonation of terminal alkyne in step 2 of cyclic mechanism. Click here to View figure |
For a true heterogeneous catalyst, the recyclability and reusability of the catalyst are important. To investigate these properties for our introduced catalyst, the reaction of benzyl bromide with phenyl acetylene was selected as a model reaction. The recovered catalyst, after drying at 50 °C was reused in the next runs. Almost consistent activity was observed over five runs. The results showed that the newly developed catalyst maintained an optimal activity after 5 repeated cycles (Table 4).
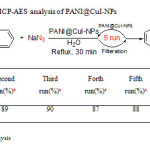 |
Table4: Reusability study and ICP-AES analysis of PANI@CuI-NPs Click here to View table |
Conclusion
We have developed a mild, simple, cost-effective and green procedure for one-pot regiospecific synthesis of 1,4-disubstituted-1H-1,2,3-triazoles via Huisgen 1,3-dipolar cycloaddition reaction between terminal alkynes, α-halo ketones or benzyl halides and NaN3 in the presence of recyclable PANI@CuI-NPs using water as reaction medium. PANI@CuI-NPs exhibited much more efficiency than unsupported bulk CuI without giving alkyne-alkyne byproduct. The reusability of this catalyst at least over five runs will makes it a useful and important addition to the previously reported catalysts.
Experimental
Materials
Aniline and other solvents were distilled before use. All other chemicals were procured from commercial sources and used as such without further purification. PANI was synthesized according to literature procedure.20
Preparation of polyaniline supported CuI nanoparticles (PANI@CuI-NPs)
PANI (1000 mg) was charged into a round-bottom flask containing an EtOH solution (25 mL) of cuprous iodide (285 mg, 1.5 mmol) and was refluxed under nitrogen atmosphere for 12 h. The resultant catalyst was filtered off and washed with acetonitrile followed by acetone. The residue was dried in air for 24 h to afford the black catalyst.
Determination of the Copper Content in PANI@CuI-NPs Catalyst
To evaluate the copper content, the PANI@CuI-NPs (100 mg) was extracted with concentrated HCl (5×2 ml) in a screw-capped vessel, followed by treatment with concentrated nitric acid (2 ml) to digest the metal complex. The mixture was then transferred into a volumetric flask (100 ml), diluted 1:50 for the second time and was analyzed by the ICP analysis. The copper content of PANI@CuI-NPs was calculated to be 179 mg of CuI per 1000 mg of polymeric catalyst (loading= 0.94 mmol.g-1)
General procedure for multicomponent synthesis of 1,4-disubstituted-1H-1,2,3-triazoles
A round-bottomed flask containing a stirrer bar was charged with benzyl halide or α-halo ketones (1.0 mmol), NaN3 (1.1 mmol), terminal alkyne (1.0 mmol), PANI@CuI-NPs (53 mg, 5 ℅), and water (5.0 ml). The reaction was followed at reflux condition and was complete after appropriate time (Table 2) as monitored by TLC analysis. After the reaction completion, the whole reaction mixture was directly passed through celite and washed with acetone (3×5 ml). The recovered catalyst was dried and stored for another consecutive reaction run. Acetone was removed by rotation evaporator under vacuum and the residue was precipitated in water. The precipitate was collected by filtration. The collected solid residue was recrystallized in ethanol or ethanol/water (1:3 v/v) and recovered by simple filtration and dried under vacuum for 24 h at room temperature.
Selected spectral data
1-benzyl-4-(thiophen-2-yl)-1H-1, 2, 3-triazole (Table 2, entry 6) 1H NMR (CDCl3, 400 MHz, TMS) 7.6- (s, 1H), 7.27-7.38 (m, 7H), 7.04- 7.35(m, 1H), 5.56 (s, 2H). 13C NMR (CDCl3, 100 MHz) 134.4, 132.8, 129.2, 128.8, 128.1, 127.6, 125.0, 124.1, 119.2, 54.3.
2-(1-Benzyl-1H-[1,2,3]triazol-4-yl)-propan-2-ol (Table 2, entry 7) bright brown solid (powder), melting point: 74-76 °C; IR: = 3298 (OH-valence), 3144 (=CH- valence), 2977-2850 (CH2,CH3-valence), 1452 (CH2,CH3-deform.), 1172 (C-O-valence), 729, 696 (=C-H-deform.) cm-1; MS (EI): m/z (%): 217 (<1) [M+], 202 (30), 170 (12), 92 (8), 91 (100), 80 (8), 65 (13); 1H NMR (200 MHz, CDCl3): δ = 7.34-7.20 (6H, m), 5.43 (2H, s), 1.66 (6H, s) ppm; 13C NMR (50 MHz, CDCl3): δ = 134.08, 128.74, 128.72, 128.47, 127.90, 30.93.
2-(1-Benzyl-1H-[1,2,3]triazol-4-yl)-but-3-en-2-ol (Table 2, entry 8) red-brown oil; IR: = 3373 (OH-valence), 2980-2855 (CH2,CH3-valence), 1456 (CH2,CH3-deform.), 1139 (C-O-valence), 718, 693 (=C-H-deform.) cm-1; MS (EI): m/z (%): 229 (<1) [M+], 212 (11), 186 (36), 92 (9), 91 (100), 65 (14); 1H NMR (400 MHz, CDCl3): δ = 7.37-7.22 (6H, m), 6.22 (1H, s), 5.45 (2H, s), 5.30 (1H, s), 5.15 (1H, s), 1.69 (3H, s) ppm, 13C NMR (100 MHz, CDCl3): δ = 143.26, 134.42, 129.10, 128.76, 128.15, 127.98, 122.10, 112.66, 54.14, 40.24, 31.59, 28.62, 22.59, 14.01 ppm.
Acknowledgment
We are thankful to Research Council of Islamic Azad University, Ahvaz branch for financial support.
References
- Fan, W. Q. and Katritzky A. R. in: Comprehensive Heterocyclic Chemistry II, Elsevier Science, Oxford, 1996, p. 1.
- Rostovtsev,V. V.; Green,L. G.; Fokin, V. V. and Sharpless, K. B. Angew. Chem. Int. Ed., 2002, 41, 2596.
- Tornøe, C. W.; Christensen, C. and Meldal, M. J. Org. Chem., 2002, 67, 3057.
- Kolb, H. C. and Sharpless, K. B. Drug Discovery Today, 2003, 8, 1128.
- Huisgen, R. Angew. Chem. Int. Ed., 1963, 2, 565.
- Aucagne, V. and Leigh, D. A. Org. Lett., 2006, 8, 4505.
- Anastas, P. T. and Warner, J. C. Green Chemistry: Theory and Practice, Oxford University Press, New York, 1998.
- Hodge, P. Ind. Eng. Chem. Res., 2005, 44, 8542.
- Galaffu, N.; Sechi, G. and Bradly, M. Mol Divers., 2005, 9, 263.
- Erb, B.; Kucma, J. P.; Mourey, S. and Struber, F. Chem. Eur. J., 2003, 9, 2582.
- Marra, A.; Vecchi, A.; Chiappe, C.; Melai, B. and Dondoni, A. J. Org. Chem., 2008, 73, 2458.
- Albadi, J.; Keshavarz, M.; Shirini, F. and Vafaie-nezhad, M. Catal. Commun., 2012, 27, 17.
- Albadi, J. and Keshavarz, M. Synth. Commun., 2013, 43, 2019.
- Albadi, J.; Keshavarz, M.; Abedini, M. and Vafaie-nezhad, M. Chin. Chem. Lett., 2012, 23, 797.
- Keshavarz, M. and Badri, R. Mol Divers., 2011, 15, 957.
- Zarei Ahmadi, A.; Heidarizadeh, F.; Keshavarz, M. Synth. Commun., 2013, 43, 2100
- Keshavarz, M.; Iravani, N.; Ghaedi,A.; Zarei Ahmadi, A.; Vafaie-nezhad, M.; Karimi, S. SpringrPlus, 2013, 2, 64.
- Keshavarz, M.; Karami, B.; Zarei Ahmadi, A.; Ghaedi, A.; Vafaei, H. C. R. Chim., 2014, 17, 570.
- Houdayer, A.; Schneider, R.; Billaud, D.; Ghanbaja, J. and Lambert, J. Appl. Organomet. Chem. 2005, 19,1239.
- Choudary, B. M.; Roy, M.; Roy, S.; Kantam, M. L.; Sreedhar B. and Kumar, K. V. Adv. Synth. Catal., 2006, 348, 1734.
- Antilla, J. C.; Baskin, J. M.; Barder T. E. and Buchwald, S. L. J. Org. Chem., 2004, 69, 5578.
- Cai, Q.; Zhu, W.; Zhang, H.; Zhang Y. and Ma, D. Synthesis, 2005, 496.

This work is licensed under a Creative Commons Attribution 4.0 International License.









