Comparison of polyamide-carbon nanotube and polyamide- nano silica composite membranes performance for purification of ethanol
Azam Marjani*, Homayon Ghanipour
Islamic Azad University, Arak Branch, Department of Chemistry, Arak, Iran
Corresponding author Email: a-marjani@iau-arak.ac.irDOI : http://dx.doi.org/10.13005/ojc/310257
Article Received on :
Article Accepted on :
Article Published : 18 Jun 2015
The pervaporation (PV) separation performance of carbon nano tube and nano silica filled polyamide membranes were compared with pure polyamide for the dehydration of ethanol. The separation of synthesized membranes was compared with each other in separation of ethanol from ethanol/water mixture via pervaporation process. Feed concentration and temperature factors effect on ethanol separation was investigated. The results demonstrated that separation factor of polyamide-carbon nano tube is approximately higher than polyamide-nano-silica. However, the permeate concentration of ethanol for polyamide-nano-silica is much better by comparison with nano tube-polyamide and pure polyamide mixed membrane membranes.
KEYWORDS:Nano mixed matrix membranes; Polyamides; Nano Silica; Nano tube; Synthesis
Download this article as:| Copy the following to cite this article: Marjani A, Ghanipour H. Comparison of polyamide-carbon nanotube and polyamide- nano silica composite membranes performance for purification of ethanol. Orient J Chem 2015;31(2). |
| Copy the following to cite this URL: Marjani A, Ghanipour H. Comparison of polyamide-carbon nanotube and polyamide- nano silica composite membranes performance for purification of ethanol. Available from: http://www.orientjchem.org/?p=9283 |
Introduction
Ethanol is a very vital and widely applied as solvent in chemical industries. However, due to the forms of azeotrope with water at one time it reaches 89.4 mol% at 351.15 K and atmospheric pressure, this mixture is difficult to be separated by using conventional distillation process and but can be conducted through azeotropic distillation. Nevertheless, azeotropic distillation is more energy consuming in comparison with conventional distillation [1-3].
A great deal of researchers has been investigated membrane processes experimentally and theoretically as alternative method for separation chemicals [4-15]. Mixed matrix polymer materials as one of membrane material have attracted much attention in connection with the rapid development of nanotechnology in the last years. The combination of individual properties of polymers and nano size fillers creates synergic characteristics for mixed matrix materials, which makes them promising materials for various applications. Among the important ones is the membrane technology [16-27].
This present study extended our previous research to separation of ethanol from water. The current research purpose is comparison separation performance of ethanol with two different nano-composite membranes.
Experimental
Chemicals
Ultramid® Polyamide: PA 6 (Nylon 6, BASF) was purchased from BASF. Carbon nanotubes (multi wall CNT, puriy>95%), nano silica and dimethylacetamide were was purchased from Merck Corporation. Ethanol (Razi Refinery) and distillated water were used in the experiments. All chemicals used were of analytical reagent grade and were used without any other treatment.
Membrane preparation
Solution-casting approach was applied to prepare the mixed matrix membranes. Exact amount of polyamide was dissolved in dimethylacetamide to make a 10 wt. % solution of polymer. The solution was mixed for 1 hour to make certain the total dissolution of polymer in solvent. Subsequently, carbon nanotubes or nano silica were added to the solution at desired values for fine dispersion of carbon nanotubes or nano silica in polymer matrix, the solution was put in the ultrasonic. The resulting solution was then casted on a glass plate by using casting knife. The membrane was then dried for 24 h at ambient pressure and temperature. To assessment the performance of synthesized membranes in purification of ethanol, an experimental pervaporation setup was conducted. Effect of feed concentration and temperature on mass flux and separation factor of ethanol was investigated. Feed solutions at different concentrations ranging from 20-90 wt. % ethanol were prepared and fed to the membrane module at a constant flow rate. The temperature of feed solution was adjusted at 22-65 °C using a constant-temperature heat bath [28-39].
Results and discussion
Effect of feed concentration
Effect of ethanol concentration in the feed on mass flux and separation factor are shown in Fig. 1. The concentration of ethanol in the feed solution changes from 20 to 90 wt. % at feed temperature of 22 ○C. As it can be seen, ethanol concentration has significant effect on permeation flux and separation factor especially at higher feed concentrations. At low concentration of ethanol (below 50 wt. %), the effect of feed concentration on separation factor is not appreciable, but the separation factor suddenly increases sharply when the concentration of ethanol in the feed solution increases to 50 wt. %. The separation flux then decreases with increasing ethanol concentration from 55 wt. % to 90 wt. %. The reason for this behavior could be due to swelling of polyamide chains with increasing ethanol concentration which results in reduction of separation factor. Therefore, the maximum separation factor can be achieved at feed concentration of 50 wt. %. In addition, the separation factor of carbon nanotube is higher than nano-silica. Fig. 2 shows the PV flux as a function of the ethanol feed concentration. The permeation flux shows different trend with increasing feed concentration. A linear increasing in permeation flux is observed when the feed concentration increases from 20 wt. % to 90 wt. %. The latter could be due to increasing driving force between membrane and feed by increasing ethanol concentration in the feed which results in enhancement of permeation flux [40-52].
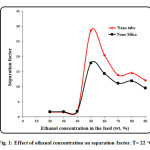 |
Figure1: Effect of ethanol concentration on separation factor. T= 22 ○C. Click here to View figure |
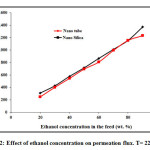 |
Figure2: Effect of ethanol concentration on permeation flux. T= 22 ○C.Click here to View figure |
Effect of feed temperature
The separation factor and permeation flux as function of feed temperature are plotted in Fig. 3. The temperature ranging from 22 to 65 ○C was considered in the experiments to obtain the effect of feed temperature. As observed, the permeation flux increases by increasing feed temperature. The mechanism of separation in pervaporation using dense membranes is the solution-diffusion mechanism. Increasing temperature would result in enhancement of solubility and diffusivity of ethanol in the polyamide membrane. On the other hand, increasing feed temperature results in reduction of separation factor (see Fig. 4). Increasing temperature causes the transport of both ethanol and water through the membrane increases and therefore separation factor decreases with increasing temperature. The maximum separation factor is obtained at feed temperature of 22 ○C [53-77].
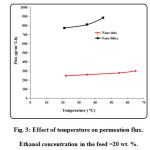 |
Figure3: Effect of temperature on permeation flux. Ethanol concentration in the feed =20 wt. %. Click here to View figure |
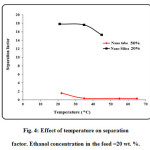 |
Figure4: Effect of temperature on separation factor. Ethanol concentration in the feed =20 wt. %. Click here to View figure |
Conclusion
In the present study, Nano particles and polyamide mixed matrix membranes were successfully prepared and their performance was investigated using PV dehydration of ethanol/water mixtures. Effect of feed concentration and temperature on permeation flux and separation factor was studied. The results showed that the membranes has the best performance at feed concentration of higher than 50 wt. %. Temperature also showed significant effect on membrane performance. Increasing feed temperature results in enhancement of permeation flux, but decreases separation factor.
Reference
- F. Barati, M. Ghadiri, R. Ghasemi, H.M. Nobari, Chem. Eng. Technol. 37 (2014) 81-86.
- A. Daraei, P. Aghasafari, M. Ghadiri, A. Marjani, Trans Indian Inst Met, 67 (2014) 223-227.
- F. Fadaei, V. Hoshyargar, S. Shirazian, S.N. Ashrafizadeh, Desalination, 284 (2012) 316.
- F. Fadaei, S. Shirazian, S.N. Ashrafizadeh, Desalination, 281 (2011) 325-333.
- F. Fadaei, S. Shirazian, S.N. Ashrafizadeh, Desalination, 275 (2011) 126-132.
- M. Fasihi, S. Shirazian, A. Marjani, M. Rezakazemi, Mathematical and Computer Modelling, 56 (2012) 278-286.
- M. Ghadiri, V. Abkhiz, M. Parvini, A. Marjani, Chem. Eng. Technol. 37 (2014) 543-550.
- M. Ghadiri, M. Asadollahzadeh, A. Hemmati, J.Water Process Eng. 6 (2015) 144-150.
- M. Ghadiri, S.N. Ashrafizadeh, Chemical Engineering and Technology, 37 (2014) 597-604.
- M. Ghadiri, S. Fakhri, S. Shirazian, Industrial and Engineering Chemistry Research, 52 (2013) 3490-3498.
- M. Ghadiri, S. Fakhri, S. Shirazian, Polymer Engineering and Science, 54 (2014) 660-666.
- M. Ghadiri, M. Ghasemi Darehnaei, S. Sabbaghian, S. Shirazian, Chemical Engineering and Technology, 36 (2013) 507-512.
- M. Ghadiri, A. Marjani, S. Shirazian, Int. J. Greenhouse Gas Control, 13 (2013) 1-8.
- M. Ghadiri, M. Parvini, M.G. Darehnaei, Poly. Eng. Sci. 54 (2014) 2222-2227.
- M. Ghadiri, S. Shirazian, Chemical Engineering and Processing: Process Intensification, 69 (2013) 57-62.
- M. Ghadiri, S. Shirazian, S.N. Ashrafizadeh, Chemical Engineering and Technology, 35 (2012) 2177-2182.
- M. Hemmati, N. Nazari, A. Hemmati, S. Shirazian, Journal of Industrial and Engineering Chemistry, 21 (2015) 1410-1416.
- V. Hoshyargar, A. Marjani, F. Fadaei, S. Shirazian, Oriental J. Chem. 28 (2012) 109-113.
- M.A. Khansary, A.H. Sani, S. Shirazian, Fluid Phase Equilibria, 385 (2015) 205-211.
- R.K. Kohnehshahri, M. Salimi, S.R.S. Mohaddecy, S. Shirazian, Oriental Journal of Chemistry, 27 (2011) 1351-1355.
- A. Marjani, P. Mohammadi, S. Shirazian, Oriental Journal of Chemistry, 28 (2012) 97-102.
- A. Marjani, M. Rezakazemi, S. Shirazian, Oriental J. Chemistry, 27 (2011) 1331-1335.
- A. Marjani, M. Rezakazemi, S. Shirazian, Oriental J. Chemistry, 28 (2012) 145-151.
- A. Marjani, Y. Shirazi, S. Shirazian, Asian Journal of Chemistry, 23 (2011) 3205-3207.
- A. Marjani, S. Shirazian, World Academy of Science, Engineering and Technology, 37 (2010) 1043-1047.
- A. Marjani, S. Shirazian, Desalination, 281 (2011) 422-428.
- A. Marjani, S. Shirazian, Oriental Journal of Chemistry, 27 (2011) 1311-1316.
- A. Marjani, S. Shirazian, Asian Journal of Chemistry, 23 (2011) 3299-3300.
- A. Marjani, S. Shirazian, Asian Journal of Chemistry, 23 (2011) 3289-3290.
- A. Marjani, S. Shirazian, Asian Journal of Chemistry, 23 (2011) 3293-3294.
- A. Marjani, S. Shirazian, Oriental Journal of Chemistry, 28 (2012) 83-87.
- A. Marjani, S. Shirazian, Oriental Journal of Chemistry, 28 (2012) 41-46.
- A. Marjani, S. Shirazian, Oriental Journal of Chemistry, 28 (2012) 23-28.
- A. Marjani, S. Shirazian, Oriental Journal of Chemistry, 28 (2012) 67-72.
- A. Marjani, S. Shirazian, Oriental Journal of Chemistry, 28 (2012) 123-129.
- A. Marjani, S. Shirazian, M. Ranjbar, M. Ahmadi, Oriental J. Chemistry, 28 (2012) 13-18.
- S.A. Miramini, S.M.R. Razavi, M. Ghadiri, S.Z. Mahdavi, S. Moradi, Chemical Engineering and Processing: Process Intensification, 72 (2013) 130-136.
- A. Moghadassi, A. Marjani, S. Shirazian, S. Moradi, Asian J. Chem. 23 (2011) 1922-1924.
- M. Mohammadi, S. Shirazian, M. Asadollahzadeh, L. Jamshidy, A. Hemmati, Polymer Engineering and Science, 55 (2015) 975-980.
- S. Moradi, Z. Rajabi, M. Mohammadi, M. Salimi, S.S. Homami, M.K. Seydei, S. Shirazian, Mathematical and Computer Modelling, 57 (2013) 1184-1189.
- F. Nosratinia, M. Ghadiri, H. Ghahremani, J. Ind. Eng. Chemistry, 20 (2014) 2958-2963.
- F. Nosratinia, H. Ghahremani, S. Shirazian, Desal. Water Treatment, 54 (2015) 1550-1555.
- M. Pishnamazi, A. Marjani, S. Shirazian, M. Samipurgiri, Oriental J. Chem. 28 (2012) 51.
- M. Ranjbar, S. Shirazian, S. Ghafarnejad Parto, M. Ahmadi, Chemical Engineering and Technology, 36 (2013) 728-732.
- S.M.R. Razavi, A. Marjani, S. Shirazian, Transport in Porous Media, 106 (2014) 323-338.
- S.M.R. Razavi, S.M.J. Razavi, T. Miri, S. Shirazian, International Journal of Greenhouse Gas Control, 15 (2013) 142-149.
- S.M.R. Razavi, S. Shirazian, M.S. Najafabadi, Poly. Eng. Sci. 55 (2015) 598-603.
- M. Rezakazemi, A. Ghafarinazari, S. Shirazian, A. Khoshsima, Polymer Engineering and Science, 53 (2013) 1272-1278.
- M. Rezakazemi, M. Iravaninia, S. Shirazian, T. Mohammadi, Polymer Engineering and Science, 53 (2013) 1494-1501.
- M. Rezakazemi, A. Marjani, S. Shirazian, Chem. Eng. Technology, 36 (2013) 483-491.
- M. Rezakazemi, Z. Niazi, M. Mirfendereski, S. Shirazian, T. Mohammadi, A. Pak, Chemical Engineering Journal, 168 (2011) 1217-1226.
- M. Rezakazemi, M. Shahverdi, S. Shirazian, T. Mohammadi, A. Pak, Chemical Engineering Journal, 168 (2011) 60-67.
- M. Rezakazemi, S. Shirazian, S.N. Ashrafizadeh, Desalination, 285 (2012) 383-392.
- S. Shirazian, S.N. Ashrafizadeh, Solvent Extraction and Ion Exchange, 28 (2010) 267-286.
- S. Shirazian, S.N. Ashrafizadeh, Separation Science and Technology, 45 (2010) 515-524.
- S. Shirazian, S.N. Ashrafizadeh, I. Engineering Chemistry Research, 50 (2011) 2245-2253.
- S. Shirazian, S.N. Ashrafizadeh, Chem. Engineering and Technology, 36 (2013) 177-185.
- S. Shirazian, S.N. Ashrafizadeh, Indu. Eng. Chemistry Research, 53 (2014) 12435-12444.
- S. Shirazian, S.N. Ashrafizadeh, Fuel, 148 (2015) 112-119.
- S. Shirazian, S.N. Ashrafizadeh, RSC Advances, 5 (2015) 30719-30726.
- S. Shirazian, S.N. Ashrafizadeh, J. Ind. Engineering Chemistry, 22 (2015) 132-137.
- S. Shirazian, F. Fadaei, S.N. Ashrafizadeh, Solv. Extrac. Ion Exchange, 30 (2012) 490-506.
- S. Shirazian, A. Marjani, F. Fadaei, Desalination, 277 (2011) 135-140.
- S. Shirazian, A. Marjani, M. Rezakazemi, Engineering with Computers, 28 (2012) 189-198.
- S. Shirazian, A. Marjanm, F. Azizmohammadi, Oriental J. Chemistry, 27 (2011) 485-490.
- S. Shirazian, A. Moghadassi, S. Moradi, Simul. Model. Practice and Theory, 17 (2009) 708.
- S. Shirazian, S.G. Parto, S.N. Ashrafizadeh, Int. J. Applied Ceramic Technol. 11 (2014) 793.
- S. Shirazian, M. Pishnamazi, M. Rezakazemi, A. Nouri, M. Jafari, S. Noroozi, A. Marjani, Chemical Engineering and Technology, 35 (2012) 1077-1084.
- S. Shirazian, M. Rezakazemi, A. Marjani, S. Moradi, Desalination, 286 (2012) 290-295.
- S. Shirazian, M. Rezakazemi, A. Marjani, M.S. Rafivahid, Asia-Pacific J. Chemical Engineering, 7 (2012) 828-834.
- M.R. Sohrabi, A. Marjani, S. Moradi, M. Davallo, S. Shirazian, J. the Chem. Society of Pakistan, 33 (2011) 464-473
- M.R. Sohrabi, A. Marjani, S. Moradi, M. Davallo, S. Shirazian, Asian J. Chem. 23 (2011) 302.
- M.R. Sohrabi, A. Marjani, S. Moradi, M. Davallo, S. Shirazian, Applied Mathematical Modelling, 35 (2011) 174-188.
- M.R. Sohrabi, A. Marjani, S. Moradi, M. Davaloo, S. Shirazian, Asian J. Chemistry, 23 (2011) 4227-4228.
- M.R. Sohrabi, A. Marjani, S. Shirazian, S. Moradi, Asian Journal of Chemistry, 23 (2011) 4229-4230.
- K. Tahvildari, A. Zarabpour, M. Ghadiri, A. Hemmati, Polymer Engineering and Science, 54 (2014) 2553-2559.
- M. Valavi, S. Shirazian, A.F. Pour, M. Ziary, J. Solution Chemistry, 42 (2013) 1423-1437.

This work is licensed under a Creative Commons Attribution 4.0 International License.









