Classification of Transition Metal Carbonyl Clusters Using the 14n Rule Derived from Number Theory
Enos Masheija Kiremire
Department of Chemistry and Biochemistry, University of Namibia, Private Bag 13301, Windhoek, Namibia
DOI : http://dx.doi.org/10.13005/ojc/310201
Article Received on :
Article Accepted on :
Article Published : 09 Jun 2015
This paper introduces a method of classifying clusters of the transition metal carbonyls and main group elements based on the 14n and 4n rules. The two rules are interrelated. They were derived from close analysis of the relationship between the number of skeletal atoms, valence electrons and eighteen electron rule in the case of transition elements, and octet rule for main group elements and the known bonds or linkages of simple clusters. The analysis reveals that there is an infinite range of carbonyl clusters some of which are yet to be discovered. These clusters occur in an orderly manner in of clusters pertaining to definite series. In addition to the categorization of clusters, tentative structures of some clusters especially the simple ones can be predicted. Furthermore, the method can identify the INNER CORE NUCLEAR CLUSTER of some large carbonyl clusters. The method is simple and enjoyable to use.
KEYWORDS:Classification; Transition; Derived
Download this article as:| Copy the following to cite this article: Kiremire E. M. Classification of Transition Metal Carbonyl Clusters Using the 14n Rule Derived from Number Theory. Orient J Chem 2015;31(2). |
| Copy the following to cite this URL: Kiremire E. M. Classification of Transition Metal Carbonyl Clusters Using the 14n Rule Derived from Number Theory. Available from: http://www.orientjchem.org/?p=9151 |
Introduction
Transition metal carbonyl clusters have stimulated immense interest for several decades largely due to their vast and intriguing structural architecture,numerous potential industrial applications and challenging bonding properties.In this regard, the work by Wade and Mingos1-2as well as Jemmis3-4 has contributed tremendously towards understanding of clusters. Subsequently, other research groups such as those headed by King, Teo,Zanello, and Slovokhotov5-9 have expanded the knowledge especially on the large carbonyl clusters. The polyhedral skeletal electron pair theory (PSEPT) which uses mainly the 4n, 5n and 6n rules has classified clusters as closo, nido, arachno, hypho and some klapo10. However on closer scrutiny of the structures and formulas of the simple boranes, hydrocarbons, carboranes and transition metal carbonyl clusters reveals that these clusters both simple and complex ones belong to a wide range of an infinite series. The series are formed according to definite numerical sequences reminiscent of the atomic number of elements.
An infinite Universe of Cluster Series
Transition metal carbonyl clusters like boranes portray an infinite number of series11.The series were deduced from the cluster table series generated using the cluster number (k-value)12. For small clusters, the k value corresponds to the number of bonds linking up the skeletal atoms that obey the 8-electron rule or the 18-electron rule. The cluster number is calculated using a simple empirical formula given by k = ½ (E-V) where E is the sum of octet electrons in case of main group elements or eighteen electrons in case of transition metals. This is summarized in Tables 1 and 2.
On closer analysis of cluster tables further revealed that the clusters portray an infinite number of clusters series based on 14n and 4n as bases for transition metals and main group elements respectively and these are shown in a selected short Table 3. Looking at table 3, it is quite clear that the series increase by steps of 2 as you go down the table or decrease in steps of 2 as you go up the table. Further scrutiny of the cluster tables11-12 indicated that the cluster number k is related to the corresponding clusters series by 2n plus or minus one-half a multiple of 2. For instance if the series S = 14n + 8 the corresponding k value will be 2n-4 while for the S = 14n-30, the k value will be 2n+15. The k values for the transition metal cluster will be the same as the corresponding main group element cluster. Selected examples showing the calculation of a cluster number using the cluster series are given in Tables 4 and 5 for main group element and transition metal carbonyl clusters.
Table1: The sample showing selected main group elements for illustrating the basis for the empirical formula k = ½ (E-V)
| Cluster | Observed number of skeletal bonds | number of skeletal elements (n) | E = 8n | Total Valence electrons (V) | k = ½ (E-V) |
| N2 | 3 | 2 | 16 | 10 | 3 |
| O2 | 2 | 2 | 16 | 12 | 2 |
| F2 | 1 | 2 | 16 | 14 | 1 |
| C2H2 | 3 | 2 | 16 | 10 | 3 |
| C2H4 | 2 | 2 | 16 | 12 | 2 |
| Cyclic C3H6 | 3 | 3 | 24 | 18 | 3 |
| Cyclic C4H4 | 6 | 4 | 32 | 20 | 6 |
| Cyclic C6H6 | 9 | 6 | 48 | 30 | 9 |
| P4 | 6 | 4 | 32 | 20 | 6 |
| Bi53+ | 9 | 5 | 40 | 22 | 9 |
Table 2: The sample showing selected Transition metal elements for illustrating the basis for the empirical formula k = ½ (E-V)
| Cluster Complex | Observed number of bonds | number of skeletal elements (n) | E = 18n |
Total Valence electrons (V)k = ½ (E-V)Mn2(CO)101236341Tc2(CO)101236341Re2(CO)101236341Co2(CO)81236341Rh2(CO)81236341Ir2(CO)81236341Fe2(CO)91236341Fe3(CO)123354483Ru3(CO)123354483Os3(CO)123354483Co4(CO)126472606Rh4(CO)126472606Ir4(CO)126472606Fe4(C)(CO)122―5472625Os4(CO)164472644Os5(CO)169590729Fe5(C)(CO)158590748Rh6(C)(CO)152―96108909Os6(CO)181261088412
Table 3: Portion of Cluster Series for Main Group Elements and Transition Metal Carbonyl Clusters
| TRANSTION METAL CARBONYL CLUSTER SERIES(14n) | MAIN GROUP CLUSTER SERIES(4n) | k SERIES | REMARKS |
| 14n-20 | 4n-20 | 2n+10 | |
| 14n-18 | 4n-18 | 2n+9 | |
| 14n-16 | 4n-16 | 2n+8 | |
| 14n-14 | 4n-14 | 2n+7 | |
| 14n-12 | 4n-12 | 2n+6 | |
| 14n-10 | 4n-10 | 2n+5 | Hexacapped Series(C6C) |
| 14n-8 | 4n-8 | 2n+4 | Pentacapped Series (C5C) |
| 14n-6 | 4n-6 | 2n+3 | Tetracapped Series(C4C) |
| 14n-4 | 4n-4 | 2n+2 | Tricapped Series(C3C) |
| 14n-2 | 4n-2 | 2n+1 | Bicapped Series (C2C) |
| 14n | 4n | 2n | Monocapped Series(C1C) |
| 14n +2 | 4n+2 | 2n-1 | Closo Series(C) |
| 14n +4 | 4n+4 | 2n-2 | Nido Series(N) |
| 14n +6 | 4n+6 | 2n-3 | Arachno Series(A) |
| 14n +8 | 4n+8 | 2n-4 | Hypho Series(H) |
| 14n +10 | 4n+10 | 2n-5 | Klapo Series( H-1) |
| 14n +12 | 4n+12 | 2n-6 | |
| 14n +14 | 4n+14 | 2n-7 | |
| 14n +16 | 4n+16 | 2n-8 | |
| 14n +18 | 4n+18 | 2n-9 | |
| 14n +20 | 4n+20 | 2n-10 |
Table 4: Selected simple clusters showing Relationship between the Cluster k value and the corresponding Series for Main Group Elements
| Cluster | Number of Skeletal elements(n) | 4n | Valence electrons(V) | Series(S) | k-series | k value |
| N2 | 2 | 8 | 10 | 4n+2 | 2n-1 | 3 |
| O2 | 2 | 8 | 12 | 4n+4 | 2n-2 | 2 |
| F2 | 2 | 8 | 14 | 4n+6 | 2n-3 | 1 |
| C2H2 | 2 | 8 | 10 | 4n+2 | 2n-1 | 3 |
| C2H4 | 2 | 8 | 12 | 4n+4 | 2n-2 | 2 |
| P4 | 4 | 16 | 20 | 4n+4 | 2n-2 | 6 |
| Bi53+ | 5 | 20 | 22 | 4n+2 | 2n-1 | 9 |
Table 5: Relationship between the Cluster k value and the corresponding Series for carbonyl clusters
| Cluster | Number of Skeletal elements(n) | 14n | Valence electrons(V) | Series(S) | Cluster Type | k-series | k value |
| Mn2(CO)10 | 2 | 28 | 34 | 14n+6 | Arachno | 2n-3 | 1 |
| Fe3(CO)12 | 3 | 42 | 48 | 14n+6 | Arachno | 2n-3 | 3 |
| Co4(CO)12 | 4 | 56 | 60 | 14n+4 | Nido | 2n-2 | 6 |
| Fe4(C)(CO)122― | 4 | 56 | 62 | 14n+6 | Arachno | 2n-3 | 5 |
| Os4(CO)16 | 4 | 56 | 64 | 14n+8 | Hypho | 2n-4 | 4 |
| Os5(CO)16 | 5 | 70 | 72 | 14n+2 | Closo | 2n-1 | 9 |
| Fe5(C)(CO)15 | 5 | 70 | 74 | 14n+4 | Nido | 2n-2 | 8 |
| Os6(CO)18 | 6 | 84 | 84 | 14n | Monocapped | 2n | 12 |
Classification of Transition metal Carbonyls Using the 14n Rule
The 14n rule is extremely useful in categorization of a cluster into its series given the formula of the cluster. This method is exceedingly simple and rapid. The idea is to compare the reference value (14n) with the total valence electrons(V) of the cluster. If 14n is less than V, then the shortfall must be added to the 14n value to be equal to V value. On the other hand if 14n value is greater than V value, the excess must be subtracted to balance the V value. In so doing a cluster series of the complex is derived. It may be represented as S = 14n ± q, where S represents the cluster series, n the number of skeletal cluster atoms, and q is a multiple of 2. This concept is illustrated in scheme 1.
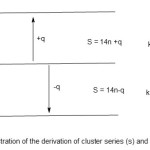 |
Scheme 1 |
A cluster number is given by k = 2n± q/2. The k value formula applies to both main group elements which obey the octet rule and transition metal skeletal elements which obey the 18-electron rule.The following selected examples are utilized for illustrations.
For example, Os3(CO)12: n = 3, 14n = 14×3 = 42. The number of valence electrons(V) = 8×3+2×12 = 48. The baseline reference 14n is less than V by 6. Hence the cluster series for the complex is S = 14n+6 and the cluster k value is k = 2n-3 = 6-3=3. The osmium atom form a triangular shape(k =3). Furthermore, the cluster belongs toArachnoseries(see Table 3). Consider OS4(CO)14; n = 4, 14n = 56, V = 60. Hence, S = 14n+4. This means that the cluster belong to the Nido series(see Table 3) and its cluster number k = 2n-2 = 6. The osmium skeletal atoms form a tetrahedral shape. Os5(CO)16; n = 5, 14n = 70 and V = 72. The cluster belongs to the series S= 14n+2. This is a member of Clososeries(see Table 3). The cluster number k = 2n-1 = 9. This corresponds to a trigonalbipyramidal geometry. For Os5(CO)19; n =5, 14n = 70 and V = 78. This gives us the series S = 14n +8 (Hypho series). The cluster number k = 2n-4 = 6. The shape of the osmium skeletal atoms is two edge-linked triangles. Consider Co6(CO)15(C)2― cluster, n = 6, 14n = 84 and V = 90. Hence, S = 14n+6. This cluster belongs to an Arachno series with cluster number k = 2n-3 = 9. This is expected to have a trigonal prism shape as is observed7. Take another example Ru6C(CO)17; n = 6, 14n = 84 and V = 86. Therefore the cluster belongs to the cluster series S = 14n+2. This a member of Closo series with k = 2n-1 = 11. This is expected to have an octahedral shape as is reported.
The Capping Process and Capping Series:-The Presence of a Nuclear Cluster Inner Core
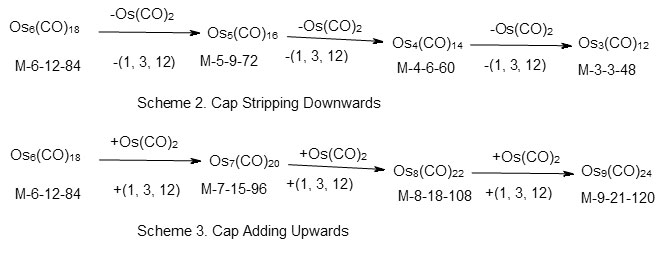
Using the series approach, the capped clusters can also easily be identified. For instance in the case of Os6(CO)18, n = 6, 14n = 84, V = 84. Hence S= 14n = C1C which belongs to mono-capped series. The cluster number k = 2n = 12. Since it has six skeletal elements, it means the capping will be on the remaining five atoms. In this coding, this is expressed as C1C[M-5]. The symbol M-5 corresponds to B5H52―closoborane ion which has a trigonalbipyramid geometry. The analysis of the series, reveals that the capping process involves a change in one skeletal atom and cluster number by 3 (∆k = 3) and valence electrons by 12. This is expressed as (1, 3, 12). In case of osmium, this is equivalent to changing a cluster by Os(CO)2 fragment since Os donates 8 valence electrons and the two CO ligands donate 4. According to the coding system we designed in our study of clusters*, the complex Os6(CO)18 can be expressed as M-6-12-84 where 6 denotes the number of cluster elements M, 12 denotes the cluster number k and 84 the number of cluster valence electrons. The capping in Os6(CO)18 cluster can be traced in a decreasing or increasing manner. This is summarized in scheme 2. The gradual increase in capping fromOs6(CO)18 cluster is shown in scheme 3. Some of the clusters shown in the scheme may not be known as this is a hypothetical model. Hence the capping from Os6(CO)18 upwards may be represented as C1C[M-5]→C2C[M-5]→C3C[M-5]→C4C[M-5]→C5C[M-5] and so on. Since, in principle, the capping of [M-5] inner core can go from 1 to 2, 3, 4, 5, 6 and so on, the inner cluster in this case [M-5] may be regarded as the Nuclear Cluster Inner Core of the complex. Many inner core cluster units have been revealed using the series number theory. Selected cluster complexes with inner core clusters are given in Table 6.
Capping the Octahedral [M-6]Clusters
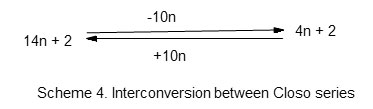
Let us consider the capping of an octahedral complex. One of the well known octahedral cluster is B6H62―. For this cluster of the main group element, n =6, 4n =24 and V = 26. Hence S = 4n+2. This means the cluster is a member of the Closo series. The six boron atoms arrange themselves in an octahedral shape (Oh). The cluster number is given by k = 2n-1 = 11 and the cluster code is M-6-11-26. The corresponding osmium cluster complex is Os6(CO)182―. In this case also, n =6, 14n = 84 and V = 86. Thus, S = 14n +2 which places the complex into the category of Closo series. The osmium skeletal atoms occupy an octahedral geometry (Oh) and the cluster number is given by 2n-1 = 11. Hence the cluster code is M-6-11-86. Comparing the cluster series (14n+2) of transition metal cluster with that of the corresponding main group series (4n +2), the difference in the number of valence electrons is 10n. Thus, (14n+2-10n = 4n +2). In the case of the six transition metal skeletal elements we can subtract (6×10 =60) from 86 valence electrons and we arrive at 26 electrons. This removal of 10 electrons from each of the 6 skeletal osmium elements is as if each osmium atom has lost a d10 electrons in so doing the cluster is transformed into that of the main group elements. Hence, M-6-11-86 removal of 60 electrons becomes M-6-11-26 and resembles the boranecloso cluster B6H62―. Alternatively, we can add 10n to the 26 electrons and get 86. This is summed up in scheme 4.
Interconversion among other corresponding series of the main group elements and transition metal carbonyl clusters can be done in the same way. This relationship is what is also expressed in Table 3. As discussed earlier, the capping step-wise constant can numerically be represented as (1, 3,12). Hence, the octahedral capping on Os6(CO)182―[M-6-11-86] can be expressed as M-6-11-86 +(1,3,12)→M-7-14-98. This will give us the mono-capped cluster as observed in Os7(CO)21. In theOs7(CO)21 cluster, n = 7, 14n =98 and V= 98. Hence the cluster series S = 14n . The cluster capping will be given by Cp = C1C. Since one of the seven skeletal atoms will get involved in capping, the rest will constitute the inner core and the symbol C1C[M-6] can be used to represent a mono-capped cluster and k = 2n =14 and the cluster code becomes M-7-14-98. Continuing the process of capping, M-7-14-98 +(1,3,12)→M-8-17-110. This is a bi-capped cluster. A representative example is Os8(CO)222―; n =8, 14n = 112, V = 110. Hence S =14n-2 and Cp =C1+C1 = C2C. Since 14n represents the first capped series, for every (-2) added represents an additional capped series. Hence S= 14n-2 series symbolically corresponds toCp= C1+C1 = C2C. This is a bi-capped series. Since 2 atoms of the 8 osmium atoms are used to form caps, the remaining six may be regarded to constitute the INNER CLUSTER CORE [M-6]. The symbol for the bi-capped octahedral cluster in this article may
be represented asCp =C2C[M-6]. For ease of identifying cluster series including those which involve capping, it is deemed fit to use the capping symbol as CxC[M-n] where x = 1,2, 3,4, 5,6,.. , M – represents the cluster element and n the number of the cluster elements in the nucleus. In fact, on deeper analysis of the series, the symbol [M-n] corresponds to a closoborane cluster. For instance, [M-4] Ξ B4H42―, [M-5] Ξ B5H52―, [M-6] Ξ B6H62― and so on.
Let us illustrate this approach to cluster categorization on some more examples for illustration. For example Os9H(CO)24― cluster, n =9, 14n = 126, V = 122. The cluster series S =14n-4. This the same as{14n-2(2)}. According to cluster series symbol, this equivalent toCp= C1+C2 = C3C[M-6]. Therefore, it is a tri-capped complex based on an octahedral nuclear core.In the case of Rh10(CO)212― cluster, n = 10, 14n = 140, V = 134. Therefore S = 14n-6→{14n-3(2)}. This gives us Cp = C1+C3 = C4C[M-6]. This means this is a tetra-capped cluster with an octahedral nuclear cluster inner core. This applies the same forOs10(CO)262―comple;, n = 10, 14n = 140 and V= 134. Hence S = 14n-6. Symbolically in terms of cluster series, this corresponds to {14n-3(2)} andCp = C1 + C3 = C4C[M-6]. This is a tetra-capped cluster on an octahedral cluster nucleus. We can take another illustration on another example, Pd6Ru6(CO)242―; n =12, 14n = 168 and V= 158. Hence S = 14n -10. In cluster series, symbolically, this corresponds to {14n-5(2)} and Cp = C1 + C5 = C6C[M-6]. Thus, the cluster is a hexa-capped octahedral as reported in literature*. An amazing result is obtained when the method is applied to Ni38Pt6(CO)48H5― carbonyl cluster. In this case, n = 44, 14n = 616, V = 542. Hence S = 14n-74. This can be expressed as {14n-37(2)}. According to cluster series, this translates into Cp= C1+ C37 = C38C[M-6]. This means that the cluster has an octahedral nuclear cluster core. The result is
indeed fascinating as an octahedral nuclear cluster comprising of six platinum atom are observed by x-ray structural analysis8.
Due to the simplicity of the method, a large number of clusters were categorized using the 14n rule. These results are given in Table 7, The table includes some of the clusters which have been categorized on the basis of their type of cluster nuclear core given in Table 6.In forming series,│∆k│ = 2 and │∆V│ = 14. In case of osmium carbonyl clusters, this corresponds to the fragment [Os(CO)3 →8+6 = 14]. However in forming capping series, │∆k│ = 3 and │∆V│ = 12. This corresponds to the fragment [Os(CO)2 →8+4 = 12]. On the basis of this information, a constructed series of osmium carbonyl clusters have been generated and are shown in Table 8. Johnsonetalhave generated similar clusters of ruthenium carbonyls in their EDESI-MS studies13.
Table6: Selected Capped Custer with Nuclear Inner cluster cores from M-2 to M-14
| CLUSTER INNER CORE | CLUSTER | CLUSTER SERIES (S) | CLUSTER k VALUE | CAPPING SERIES (Cp) |
| M-2 | Ru8Pt(CO)192― | 14n-12 | 2n+6 | C7C[M-2] |
| Os17(CO)362― | 14n-28 | 2n+14 | C15C[M-2] | |
| Pd35(CO)23L15 | 14n-64 | 2n+32 | C33C[M-2] | |
| M-4 | RuOs5(CO)14(C6H6) | 14n-2 | 2n+1 | C2C[M-4] |
| Cu2Ru6(C)(CO)16L2 | 14n-6 | 2n+3 | C4C[M-4] | |
| Pt19(CO)224― | 14n-28 | 2n+14 | C15C[M-4] | |
| Pd23(CO)20L8 | 14n-36 | 2n+18 | C19C[M-4] | |
| Pd28Pt13L13H12(CO)27 | 14n-72 | 2n+36 | C37C[M-4] | |
| M-5 | Os6(CO)18 | 14n | 2n | C1C[M-5] |
| Au3Ru4(CO)12L3H | 14n-2 | 2n+1 | C2C[M-5] | |
| Os6Pt2(CO)16(COD)2, COD =2e donor | 14n-4 | 2n+2 | C3C[M-5] | |
| Rh9(CO)193― | 14n-6 | 2n+3 | C4C[M-5] | |
| Os7(CO)19Au2L2 L2 = Ph2PCH2CH2PPh2 | 14n-6 | 2n+3 | C4C[M-5] | |
| Ru8Pt2(CO)23H2 | 14n-8 | 2n+4 | C5C[M-5] | |
| Ru7Pt3(CO)22H2 | 14n-8 | 2n+4 | C5C[M-5] | |
| Rh14(CO)254― | 14n-16 | 2n+8 | C9C[M-5] | |
| Rh15(CO)273― | 14n-18 | 2n+9 | C10C[M-5] | |
| M-6 | Os7(CO)21 | 14n | 2n | C1C[M-6] |
| Re7(C)(CO)213― | 14n | 2n | C1C[M-6] | |
| Rh7(CO)163― | 14n | 2n | C1C[M-6] | |
| Os7H2(CO)20 | 14n | 2n | C1C[M-6] | |
| Re7(C)(CO)213― | 14n | 2n | C1C[M-6] | |
| Os6Pt2(CO)17L4 | 14n-2 | 2n+1 | C2C[M-6] | |
| Os8(CO)222― | 14n-2 | 2n+1 | C2C[M-6] | |
| Ru8H2(CO)212― | 14n-2 | 2n+1 | C2C[M-6] | |
| Os8H(CO)22― | 14n-2 | 2n+1 | C2C[M-6] | |
| Os8(C)(CO)21 | 14n-2 | 2n+1 | C2C[M-6] | |
| Re8(C)(CO)242― | 14n-2 | 2n+1 | C2C[M-6] | |
| Os9(CO)242― | 14n-4 | 2n+2 | C3C[M-6] | |
| Os9H(CO)24― | 14n-4 | 2n+2 | C3C[M-6] | |
| Rh9(CO)193― | 14n-4 | 2n+2 | C3C[M-6] | |
| Pt3Ru6(C)(CO)16L4 | 14n-4 | 2n+2 | C3C[M-6] | |
| PtRh8(CO)192― | 14n-4 | 2n+2 | C3C[M-6] | |
| Ru10(C)(CO)242― | 14n-6 | 2n+3 | C4C[M-6] | |
| Os10(CO)262― | 14n-6 | 2n+3 | C4C[M-6] | |
| Os10(C)(CO)242― | 14n-6 | 2n+3 | C4C[M-6] | |
| Os10(H)4(CO)242― | 14n-6 | 2n+3 | C4C[M-6] | |
| Rh10(CO)212― | 14n-6 | 2n+3 | C4C[M-6] | |
| Pd6Ru6(CO)242― | 14n-10 | 2n+5 | C6C[M-6] | |
| Ir12(CO)242― | 14n-10 | 2n+5 | C6C[M-6] | |
| Rh13H3(CO)242― | 14n-12 | 2n+6 | C7C[M-6] | |
| Rh13H2(CO)243― | 14n-12 | 2n+6 | C7C[M-6] | |
| Rh13H(CO)244― | 14n-12 | 2n+6 | C7C[M-6] | |
| Ir14(CO)272― | 14n-14 | 2n+7 | C8C[M-6] | |
| Os10(C)(CO)24Au(AuL)3, L=PPh3 | 14n-14 | 2n+7 | C8C[M-6] | |
| Pd16Ni4(CO)22L22―, L=PPh3 | 14n-26 | 2n+13 | C14C[M-6] | |
| Os12Rh9(Cl)(CO)44 | 14n-28 | 2n+14 | C15C[M-6] | |
| Pd23(CO)20L10, L=PEt3 | 14n-32 | 2n+16 | C17C[M-6] | |
| Pt24(CO)302― | 14n-34 | 2n+17 | C18C[M-6] | |
| Pt26(CO)322― | 14n-38 | 2n+19 | C20C[M-6] | |
| Pd29(CO)28L72― | 14n-44 | 2n+22 | C23C[M-6] | |
| Pt38(CO)442― | 14n-62 | 2n+31 | C32C[M-6] | |
| Pd33Ni9(CO)41L64― | 14n-70 | 2n+35 | C36C[M-6] | |
| Ni38Pt6(CO)48H5― | 14n-74 | 2n+37 | C38C[M-6] | |
| M-7 | Ir9(CO)203― | 14n-2 | 2n+1 | C2C[M-7] |
| Ru6Pt3(CO)21H4 | 14n-2 | 2n+1 | C2C[M-7] | |
| Ir9(CO)19 H4― | 14n-2 | 2n+1 | C2C[M-7] | |
| Ir3Ni6(CO)173― | 14n-2 | 2n+1 | C2C[M-7] | |
| Os6Pt4(CO)22L2 | 14n-4 | 2n+2 | C3C[M-7] | |
| Os11(C)(CO)272― | 14n-6 | 2n+3 | C4C[M-7] | |
| Rh11(CO)233― | 14n-6 | 2n+3 | C4C[M-7] | |
| Ru6Pt3(AuL)2(CO)21H2 | 14n-6 | 2n+3 | C4C[M-7] | |
| Rh12(CO)25H2 | 14n-8 | 2n+4 | C5C[M-7] | |
| Os11(C)(CO)27(CuL)―, L =NCMe | 14n-8 | 2n+4 | C5C[M-7] | |
| Fe6Pd6(CO)24H3― | 14n-8 | 2n+4 | C5C[M-7] | |
| Pt3Ru10(C)2(CO)262― | 14n-10 | 2n+5 | C6C[M-7] | |
| Ni14Pt10(CO)304― | 14n-32 | 2n+16 | C17C[M-7] | |
| Ni24Pt14(CO)444― | 14n-60 | 2n+30 | C31C[M-7] |
| M-8 | Ru10(C)(CO)242― | 14n-2 | 2n+1 | C2C[M-8] |
| Ir12(CO)262― | 14n-6 | 2n+3 | C4C[M-8] | |
| Pd23(CO)22L10, L = PEt3 | 14n-28 | 2n+14 | C15C[M-8] | |
| M-9 | Ni11(C)2(CO)154― | 14n-2 | 2n+1 | C2C[M-9] |
| Co3Ni9(C)(CO)203― | 14n-4 | 2n+2 | C3C[M-9] | |
| Ni12(C)2(CO)162― | 14n-4 | 2n+2 | C3C[M-9] | |
| Co2Ni10(C)(CO)202― | 14n-4 | 2n+2 | C3C[M-9] | |
| Co13(C)2(CO)243― | 14n-6 | 2n+3 | C4C[M-9] | |
| Rh14(N)2(CO)252― | 14n-8 | 2n+4 | C5C[M-9] | |
| Rh15(C)2(CO)28― | 14n-10 | 2n+5 | C6C[M-9] | |
| Pd13Ni13(CO)344― | 14n-32 | 2n+16 | C17C[M-9] | |
| M-10 | Rh12(C)2(CO)234― | 14n-2 | 2n+1 | C2C[M-10] |
| Rh12(C)2(CO)242― | 14n-2 | 2n+1 | C2C[M-10] | |
| Rh12(C)2(CO)25 | 14n-2 | 2n+1 | C2C[M-10] | |
| Ag13Fe8(CO)323― | 14n-20 | 2n+10 | C11C[M-10] | |
| M-11 | Fe6Ni6(N)2(CO)242― | 14n | 2n | C1C[M-11] |
| Os18Hg2(C)2(CO)424― | 14n-16 | 2n+8 | C9C[M-11] | |
| Rh28(N)4(CO)41H44― | 14n-32 | 2n+16 | C17C[M-11] | |
| Au6Ni32(CO)446― | 14n-52 | 2n+26 | C27C[M-11] | |
| M-13 | Co14(N)3(CO)263― | 14n | 2n | C1C[M-13] |
| Rh15(C)2(CO)303― | 14n-2 | 2n+1 | C2C[M-13] | |
| Rh17(S)2(CO)323― | 14n-6 | 2n+3 | C4C[M-13] | |
| M-14 | Rh23(CO)38(N)4 3― | 14n-16 | 2n+8 | C9C[M-14] |
Table 7: Selected examples of carbonyl clusters categorized using the 14n rule
| 14n +8 (HYPHO); k = 2n-4 | 14n +6 (ARACHNO); k =2n-3 | 14n +4 (NIDO); k =2n-2 |
| Mn3(CO)14―(50, 2) |
Os5(CO)19(78, 6)
Co6(P)(CO)16―(92, 8)
Ni8L6(CO)8(120, 12), L = PPh3Mn2(CO)10(34, 1)
Ni2(Cp)2(CO)2(34, 1)
Os3(CO)12(48, 3)
Fe4(C)(CO)13(62, 5)
Rh6(C)(CO)152―(90, 9)
Ni8(C)(CO)162―(118, 13)Rh2(Cp)2(CO)2(32, 2)
Co4(CO)12(60,6)
Fe5(C)(CO)15(74, 8)
Os6Pt(CO)18H8(102, 12)
Ni9(C)(CO)172―(130, 16)14n+2 (CLOSO); k = 2n-114n(C1C) MONOCAP; k = 2n14n-2(C2C) BICAP; k =2n+1Re2(Cp)2(CO)3(30, 3)
Ru4(CO)12H2(58, 7)
Os5(CO)16(72, 9)
Os6(CO)182―(86, 11); Ohmingos
Os7H2(CO)19(110, 13)
Co7(N)(CO)152―(100, 13)
Rh8(C)(CO)19(114,15)
Rh10(S)(CO)222―(142, 19)
Rh12(Sb)(CO)273―(170, 23)Ru4(CO)12H4(56, 8)
Os6(CO)18(84, 12)
Cu3Fe3(CO)123―(84, 12)
Os7(CO)21(98, 14)
Rh7(CO)163―(98, 14)
Pt2Ru5(C)(CO)152―(98,14)
Co11N2(CO)213―(154, 22)
Fe6Ni6N2(CO)242―(168, 24)
Rh12C2(CO)242―(168, 24)
Pt3Ru10C2(CO)312―(182, 26)
Pt3Os4(CO)11L6(96,15), L =2e donor
Os8(CO)222―(110, 17)
Re8(C)(CO)242―(110,17)
Os6Pt2(CO)17L4(110,17)
Ru8H2(CO)212―(110, 17)
Ir9(CO)203―(124, 19)
Ru6Pt3(CO)21H3―(124, 19)
Ru10C2(CO)242―(138,21)
Co3Ni7C2(CO)153―(138, 21)
Rh12(CO)25C2(166, 25)
14n-4 (C3C) TRICAP; k = 2n +214n-6 (C4C) TETRACAP; k =2n+314n-8 (C5C) PENTACAP; k =2n+4
| 14n-10 (C6C) HEXACAP; k = 2n+5 | 14n-12 (C7C); k =2n+6 | 14n-14 (C8C) ; k =2n+7 |
| Ir12(CO)242―(158, 29) |
Pt3Ru10C2(CO)262―(172, 31)
Rh15C2(CO)28―(200,35)
Rh13(CO)24H23―(170, 32)
Rh13(CO)24H23―(170, 32)
Rh13(CO)24H4―(170, 32)Ir14(CO)272―(182, 35)
14n-16(C9C); k = 2n+814n-18(C10C); k =2n+914n-20(C11C); k =2n+10Rh14(CO)254―(180, 36)
Ir14(CO)27(180, 36)
Rh14(CO)262―(180, 36)
Os10(CO)24(AuL)4(180, 36), L = PPh2Me
Os18Hg2(CO)42C24―(264, 48)
[Os10(CO)24(C)]2Hg2―(278, 50)
Rh15(CO)273―(192, 39)
Rh23(CO)38N4―(304, 55)Pd16(CO)13L9(204, 42)
Ag13Fe8(CO)323―(274, 52)
Hg3[Ru9(C)(CO)21]22―(274, 52)
14n-22(C12C); k =2n+1114n-24(C13C); k =2n+1214n-26(C14C); k =2n+13Rh17(CO)303―(216, 45)
Os18Hg2(CO)40C22―(258, 51) Pd16Ni4(CO)22L42―(254, 53)
L = PPh3
Os18Pd3C2(CO)422―(268, 55)
Ni32C6(CO)366―(422, 77)14n-28(C15C); k = 2n+1414n-30(C16C) ; k = 2n+1514n-32(C17C) ; k = 2n+16Os17(CO)362―(210, 48)
Pt19(CO)224―(238, 52)
Pd23(CO)22L10(294, 60), L =PEt3
Rh22(CO)374―(276, 60)
Pt4Rh18(CO)354―(276, 60)
Ni14Pt10(CO)304―(304, 64)
Pd13Ni13(CO)344―(332, 68)
Rh28(CO)41H2N44―(360, 72)14n-38(C20C) ; k = 2n+1914n-44(C23C) ; k = 2n+2214n-64(C33C) ; k = 2n+32Os20(CO)402―(242, 59)
Pt26(CO)322―(326, 71)
HNi38(CO)42C65―(494, 95)
Pd29(CO)28L72―(362, 80)
L = PPh3
Pd16Ni16(CO)404―(404, 86)
Pd29Ni3(CO)404―(404, 86)Pd34(CO)24L12(412, 100)
Pd35(CO)23L15(426, 102)
Table 8: Constructed table of Osmium Carbonyl Clusters based on Simple Number Theory
| HYPHO | ARACHNO | NIDO | CLOSO | MONOCAP | BICAP | TRICAP | TETRACAP |
| Os2(CO)3 | |||||||
| Os2(CO)4 | Os3(CO)6 | ||||||
| Os2(CO)5 | Os3(CO)7 | Os4(CO)9 | |||||
| Os2(CO)6 | Os3(CO)8 | Os4(CO)10 | Os5(CO)12 | ||||
| Os2(CO)7 | Os3(CO)9 | Os4(CO)11 | Os5(CO)13 | Os6(CO)15 | |||
| Os2(CO)8 | Os3(CO)10 | Os4(CO)12 | Os5(CO)14 | Os6(CO)16 | Os7(CO)18 | ||
| Os2(CO)9 | Os3(CO)11 | Os4(CO)13 | Os5(CO)15 | Os6(CO)17 | Os7(CO)19 | Os8(CO)21 | |
| Os2(CO)10 | Os3(CO)12 | Os4(CO)14 | Os5(CO)16 | Os6(CO)18 | Os7(CO)20 | Os8(CO)22 | Os9(CO)24 |
| Os3(CO)13 | Os4(CO)15 | Os5(CO)17 | Os6(CO)19 | Os7(CO)21 | Os8(CO)23 | Os9(CO)25 | Os10(CO)27 |
| Os4(CO)16 | Os5(CO)18 | Os6(CO)20 | Os7(CO)22 | Os8(CO)24 | Os9(CO)26 | Os10(CO)28 | Os11(CO)30 |
| HYPHO | ARACHNO | NIDO | CLOSO | MONOCAP | BICAP | TRICAP | TETRACAP |
| Os2(CO)10 | Os3(CO)12 | Os4(CO)14 | Os5(CO)16 | Os6(CO)18 | Os7(CO)20 | Os8(CO)22 | Os9(CO)24 |
| Os3(CO)13 | Os4(CO)15 | Os5(CO)17 | Os6(CO)19 | Os7(CO)21 | Os8(CO)23 | Os9(CO)25 | Os10(CO)27 |
| Os4(CO)16 | Os5(CO)18 | Os6(CO)20 | Os7(CO)22 | Os8(CO)24 | Os9(CO)26 | Os10(CO)28 | Os11(CO)30 |
| Os5(CO)19 | Os6(CO)21 | Os7(CO)23 | Os8(CO)25 | Os9(CO)27 | Os10(CO)29 | Os11(CO)31 | Os12(CO)33 |
| Os6(CO)22 | Os7(CO)24 | Os8(CO)26 | Os9(CO)28 | Os10(CO)30 | Os11(CO)32 | Os12(CO)34 | Os13(CO)36 |
| Os7(CO)25 | Os8(CO)27 | Os9(CO)29 | Os10(CO)31 | Os11(CO)33 | Os12(CO)35 | Os13(CO)37 | Os14(CO)39 |
| Os8(CO)28 | Os9(CO)30 | Os10(CO)32 | Os11(CO)34 | Os12(CO)36 | Os13(CO)38 | Os14(CO)40 | Os15(CO)42 |
| Os9(CO)31 | Os10(CO)33 | Os11(CO)35 | Os12(CO)37 | Os13(CO)39 | Os14(CO)41 | Os15(CO)43 | Os16(CO)45 |
| Os10(CO)34 | Os11(CO)36 | Os12(CO)38 | Os13(CO)40 | Os14(CO)42 | Os15(CO)44 | Os16(CO)46 | Os17(CO)48 |
| Os11(CO)37 | Os12(CO)39 | Os13(CO)41 | Os14(CO)43 | Os15(CO)45 | Os16(CO)47 | Os17(CO)49 | Os18(CO)51 |
| Os12(CO)40 | Os13(CO)42 | Os14(CO)44 | Os15(CO)46 | Os16(CO)48 | Os17(CO)50 | Os18(CO)52 | Os19(CO)54 |
| Os13(CO)43 | Os14(CO)45 | Os15(CO)47 | Os16(CO)49 | Os17(CO)51 | Os18(CO)53 | Os19(CO)55 | Os20(CO)57 |
| Os14(CO)46 | Os15(CO)48 | Os16(CO)50 | Os17(CO)52 | Os18(CO)54 | Os19(CO)56 | Os20(CO)58 | Os21(CO)60 |
| Os15(CO)49 | Os16(CO)51 | Os17(CO)53 | Os18(CO)54 | Os19(CO)57 | Os20(CO)59 | Os21(CO)61 | Os22(CO)63 |
| Os16(CO)52 | Os17(CO)54 | Os18(CO)56 | Os19(CO)57 | Os20(CO)60 | Os21(CO)62 | Os22(CO)64 | Os23(CO)66 |
| Os17(CO)55 | Os18(CO)57 | Os19(CO)59 | Os20(CO)60 | Os21(CO)63 | Os22(CO)65 | Os23(CO)67 | Os24(CO)69 |
| Os18(CO)58 | Os19(CO)60 | Os20(CO)62 | Os21(CO)63 | Os22(CO)66 | Os23(CO)68 | Os24(CO)70 | Os25(CO)72 |
| Os19(CO)61 | Os20(CO)63 | Os21(CO)65 | Os22(CO)66 | Os23(CO)69 | Os24(CO)71 | Os25(CO)73 | Os26(CO)75 |
| Os20(CO)64 | Os21(CO)66 | Os22(CO)68 | Os23(CO)69 | Os24(CO)72 | Os25(CO)74 | Os26(CO)76 | Os27(CO)78 |
| Os21(CO)67 | Os22(CO)69 | Os23(CO)71 | Os24(CO)72 | Os25(CO)75 | Os26(CO)77 | Os27(CO)79 | Os28(CO)81 |
| Os22(CO)70 | Os23(CO)72 | Os24(CO)74 | Os25(CO)75 | Os26(CO)78 | Os27(CO)80 | Os28(CO)82 | Os29(CO)84 |
| Os23(CO)73 | Os24(CO)75 | Os25(CO)77 | Os26(CO)78 | Os27(CO)81 | Os28(CO)83 | Os29(CO)85 | Os30(CO)87 |
| Os24(CO)76 | Os25(CO)78 | Os26(CO)80 | Os27(CO)81 | Os28(CO)84 | Os29(CO)86 | Os30(CO)88 | Os31(CO)90 |
| Os25(CO)79 | Os26(CO)81 | Os27(CO)83 | Os28(CO)84 | Os29(CO)87 | Os30(CO)89 | Os31(CO)91 | Os32(CO)93 |
| Os26(CO)82 | Os27(CO)84 | Os28(CO)86 | Os29(CO)87 | Os30(CO)90 | Os31(CO)91 | Os32(CO)94 | Os33(CO)96 |
| Os27(CO)85 | Os28(CO)87 | Os29(CO)89 | Os30(CO)90 | Os31(CO)93 | Os32(CO)94 | Os33(CO)97 | Os34(CO)99 |
| Os28(CO)88 | Os29(CO)90 | Os30(CO)92 | Os31(CO)93 | Os32(CO)96 | Os33(CO)97 | Os34(CO)100 | Os35(CO)102 |
| Os29(CO)91 | Os30(CO)93 | Os31(CO)95 | Os32(CO)96 | Os33(CO)99 | Os34(CO)100 | Os35(CO)103 | Os36(CO)105 |
| Os30(CO)94 | Os31(CO)96 | Os32(CO)98 | Os33(CO)99 | Os34(CO)102 | Os35(CO)103 | Os36(CO)106 | Os37(CO)108 |
| TETRACAP | PENTACAP | HEXACAP | HEPTACAP | OCTACAP | NONACAP | DECACAP |
| Os9(CO)24 | Os10(CO)26 | Os11(CO)28 | Os12(CO)30 | Os13(CO)32 | Os14(CO)34 | Os15(CO)35 |
| Os10(CO)27 | Os11(CO)29 | Os12(CO)31 | Os13(CO)33 | Os14(CO)35 | Os15(CO)37 | Os16(CO)39 |
| Os11(CO)30 | Os12(CO)32 | Os13(CO)34 | Os14(CO)36 | Os15(CO)38 | Os16(CO)40 | Os17(CO)42 |
| Os12(CO)33 | Os13(CO)35 | Os14(CO)37 | Os15(CO)39 | Os16(CO)41 | Os17(CO)43 | Os18(CO)45 |
| Os13(CO)36 | Os14(CO)38 | Os15(CO)40 | Os16(CO)42 | Os17(CO)44 | Os18(CO)46 | Os19(CO)48 |
| Os14(CO)39 | Os15(CO)41 | Os16(CO)43 | Os17(CO)45 | Os18(CO)47 | Os19(CO)49 | Os20(CO)51 |
| Os15(CO)42 | Os16(CO)44 | Os17(CO)46 | Os18(CO)48 | Os19(CO)50 | Os20(CO)52 | Os21(CO)54 |
| Os16(CO)45 | Os17(CO)47 | Os18(CO)49 | Os18(CO)51 | Os19(CO)53 | Os20(CO)55 | Os21(CO)57 |
| Os17(CO)48 | Os18(CO)50 | Os19(CO)52 | Os20(CO)54 | Os21(CO)56 | Os22(CO)58 | Os23(CO)60 |
| Os18(CO)51 | Os19(CO)53 | Os20(CO)55 | Os21(CO)57 | Os22(CO)59 | Os23(CO)61 | Os24(CO)63 |
| Os19(CO)54 | Os20(CO)56 | Os21(CO)58 | Os22(CO)60 | Os23(CO)62 | Os24(CO)64 | Os25(CO)66 |
| Os20(CO)57 | Os21(CO)59 | Os22(CO)61 | Os23(CO)63 | Os24(CO)65 | Os25(CO)67 | Os26(CO)69 |
| Os21(CO)60 | Os22(CO)62 | Os23(CO)64 | Os24(CO)66 | Os25(CO)68 | Os26(CO)70 | Os27(CO)72 |
| Os22(CO)63 | Os23(CO)65 | Os24(CO)67 | Os25(CO)69 | Os26(CO)71 | Os27(CO)73 | Os28(CO)75 |
| Os23(CO)66 | Os24(CO)68 | Os25(CO)70 | Os26(CO)72 | Os27(CO)74 | Os28(CO)76 | Os29(CO)78 |
| Os24(CO)69 | Os25(CO)71 | Os26(CO)73 | Os27(CO)75 | Os28(CO)77 | Os29(CO)79 | Os30(CO)81 |
| Os25(CO)72 | Os26(CO)74 | Os27(CO)76 | Os28(CO)78 | Os29(CO)80 | Os30(CO)82 | Os31(CO)84 |
| Os26(CO)75 | Os27(CO)77 | Os28(CO)79 | Os29(CO)81 | Os30(CO)83 | Os31(CO)85 | Os32(CO)87 |
| Os27(CO)78 | Os28(CO)80 | Os29(CO)82 | Os30(CO)84 | Os31(CO)86 | Os32(CO)88 | Os33(CO)90 |
| Os28(CO)81 | Os29(CO)83 | Os30(CO)85 | Os31(CO)87 | Os32(CO)89 | Os33(CO)91 | Os34(CO)93 |
| Os29(CO)84 | Os30(CO)86 | Os31(CO)88 | Os32(CO)90 | Os33(CO)92 | Os34(CO)94 | Os35(CO)96 |
| Os30(CO)87 | Os31(CO)89 | Os32(CO)91 | Os33(CO)93 | Os34(CO)95 | Os35(CO)97 | Os36(CO)99 |
| Os31(CO)90 | Os32(CO)92 | Os33(CO)94 | Os34(CO)96 | Os35(CO)98 | Os36(CO)100 | Os37(CO)102 |
| Os32(CO)93 | Os33(CO)95 | Os34(CO)97 | Os35(CO)99 | Os36(CO)101 | Os37(CO)103 | Os38(CO)105 |
| Os33(CO)96 | Os34(CO)98 | Os35(CO)100 | Os36(CO)102 | Os37(CO)104 | Os38(CO)106 | Os39(CO)108 |
| Os34(CO)99 | Os35(CO)101 | Os36(CO)103 | Os37(CO)105 | Os38(CO)107 | Os39(CO)109 | Os40(CO)111 |
| Os35(CO)102 | Os36(CO)104 | Os37(CO)106 | Os38(CO)108 | Os39(CO)110 | Os40(CO)112 | Os41(CO)114 |
| Os36(CO)105 | Os37(CO)107 | Os38(CO)109 | Os39(CO)111 | Os40(CO)113 | Os41(CO)115 | Os42(CO)117 |
| Os37(CO)108 | Os38(CO)110 | Os39(CO)112 | Os40(CO)114 | Os41(CO)116 | Os42(CO)118 | Os43(CO)120 |
Further Highlights on Transition Metal Carbonyl Clusters
Just as mononuclear transition metal carbonyl clusters such as Ni(CO)4 and Fe(CO)5 tend to obey the 18 electron rule, the transition metal carbonyl clusters such as Mn2(CO)10, Os3(CO)12 and Ir4(CO)12 tend to do the same. Hidden in the 18 electron rule is the attainment of the noble gas configuration. If we envisage a situation in which the CO ligands stealthily donate their electrons to the cluster skeleton atoms to enable them satisfy the 18 electron rule and go away, then in small clusters,the cluster number k would represent bonds or linkages. For instance k= 1 for Cl2 giving a linear molecule and Mn2(CO)10 with k =1, the ‘Mn2’ fragment will have a single bond or linkage. Also Rh2(CP*)2(CO)2, O2, C2H4 with k = 2, the Rh2 fragment, O2 and C2 fragment each is doubly bonded. The C2H2, and W2(CO)4(CpMe)2 with k =3, the fragments C2 and W2 are each triply bonded. Likewise, Os3(CO)12, cyclopropane,C3H6 with k =3 have the fragments Os3 and C3 forming triangular shapes. The P4 molecule and Ir4(CO)12 have k= 6 and both the P4 molecule and the Ir4 fragment adopt a tetrahedral geometry. Furthermore, Os5(CO)16 and Bi53+have k= 9 and both the bismuth cluster ion and O5 fragment adopt a trigonalbipyramid shape.
As the number of skeletal atoms in transition metal carbonyl clusters increases, the k value appear not to correspond to the skeletal linkages. For instance, in Os6(CO)182― and B6H62―, k =11 and both O6 and B6 fragments have an octahedral geometry. In such cases, it is better to regard the k value as simply a cluster number characteristic of the cluster as a member of the cluster series. The k value of 11 in this case is a number within the closo cluster series S = 14n+2 and k = 2n-1 with k values (∆k =2), 1,3, 5, 7, 9, 11, 13, 15, 17, .. and so forth. For the capping series, ∆k =3 and so for the octahedral closo value of k =11, will belong to the series k =2, 5, 8, 11,14, 17, 20, 23, .. and so on. In the coding system adopted in this article the capping is defined to as to commence after the closo series. In this particular case, k = 14 will represent a monocap (C1C on an octahedral geometry,[M-6]), k = 17 a bicap(C2C[M-6]), k =20 a tricap(C1C[M-6]) and so on.
Applying the 14n rule, carbonyl clusters from simple to medium to very complex ones can readily be categorized. In some cases, the k value can be utilized as a guide to predict the cluster geometry. Just as atoms and cells of plants and animals have nuclei, the closo unit in clusters around which the capping takes place may be considered to behave as a nuclear cluster inner core. In the complexes, Pd6Ru6(CO)242―{C6C[M-6]} and Ni38Pt6(CO)48H5―{C38C[M-6]} are both centered around an octahedral closo nucleus, while Os6(CO)18{C1C[M-5]} and Ru7Pt3(CO)22H2{C5C[M-5]} are based on a closotrigonalbipyamid central core cluster unit. Using the 14n rule approach such cluster nuclei can readily be identified for a large number of clusters. The 4n rule is most appropriately utilized in categorization of clusters of the main group elements for systems which obey octet rule. The 14n and 4n rules of classifying clusters complements the existing methods currently being applied.
The Origin of the Empirical Formula k = ½ (E-V) and the Cluster Series
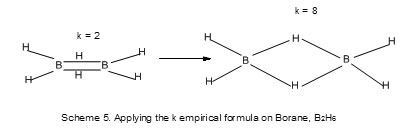
As can be seen, the application of the empirical formula, 14n and 4n rules to clusters is smooth, simple and straight forward. Therefore, it is extremely important to briefly explain their origin. In an attempt to find a simple equation to enable one easily draw a Lewis structure of simple known covalent molecules and ions, the empirical formula k = ½ (E-V) was born. Starting with N2 molecule which has a triple bond and a lone pair on each electron and each atom obeying the octet rule, the sum of hypothetical octet electrons E= 2×8 = 16 and the sum of the valence electrons V = 2×5 = 10. Hence k = ½ (E-V) = 3, the same as the triple bond of the N2molecule. Once the number of bonds 3 in this case for N2 have been obtained the calculation representing six valence electrons, the remaining 4 constitute 2 lone pairs which are evenly distributed around the two nitrogen atoms giving rise to the needed Lewis structure of N2.Using the same empirical formula, k =2 for O2 and k =1 for F2 and the Lewis structures can be obtained in the same manner. What is surprising, the empirical formula gave a k value of 4 for C2 molecule in agreement with the recent reports* meaning that C2 has a quadruple bond. This contradicts the molecular energy level diagram conventional result of C2which gives a double bond.Table 1 gives more selected examples. When the empirical formula is applied to C2H2, E = 2X8 = 16 for the two carbon atoms which obey the octet rule and V = 2×4+2 = 10 , the sum of the valence electrons. The two hydrogen atoms are considered as donating additional electrons to assist the two carbon atoms satisfy the octet rule. The k value for the fragment ‘C2’ in C2H2 is 3. This corresponds to the triple bond in the molecule. What is interesting is that if we assume that each of the hydrogen atoms in C2H2 also obeys the two(2) electron rule of [He] configuration, then the refined E for C2H2 will be 2×8+2×2= 20 and V= 10. The refined k value for C2H2 will be equal to ½ (20-10) = 5. This means all the bonds in C2H2 will be five including the triple bond. This is in agreement with the structure of C2H2. Using the same approach, the unrefined k value for C2H4 is 2 and the refined one 6. This corresponds to the structure of C2H4. Diborane, B2H6 is isoelectronic to C2H4. When the empirical formula k = ½ (E-V) is applied to it, a value of 2 is obtained. Since the boron atom is electron deficient to satisfy the octet rule in the case of B2H6 unlike the C2H4 the additional two hydrogens may be considered to be donors of electrons to the double bond to enable the two boron atoms satisfy the octet rule. The calculation also gives a refined k value of 8. This is in agreement with the diborane banana bonds structure of diborane as is currently known. This simple calculation underpins the power of noble gas configuration and by implication the octet rule and now the helium rule for hydrogen atom.
A coding for the molecules showing the number of atoms, bonds and electrons in a given molecular formula was designed. The code is represented as (M-n-k-V) where M represents the atom that obeys the octet rule, k is the cluster number or bonds in the case of simple molecules and V is the total valence electrons. Analyzing this coding for molecules, the cluster series were developed. In the case of C2 this becomes M-2-4-8. Arising from this code, it is clear that there is a relationship between the number of atoms(n) and valence electrons(V). This relationship is V= 4n =8 or in terms of series, S = 4n and k = 2n = 4. For the N2 molecule, the code is M-2-3-10. In this case, V= 4n+2 = 10 and this gives us the series S = 4n+2 and k =2n-1. For the O2 molecule, the code is M-2-2-12. This gives us the series code of S= 4n+4 and k = 2n-2 and the F2 molecule, the code is M-2-2-14 from which S = 2n+6 and k = 2n-3.
In the case of transition metal carbonyl clusters, E represents the sum of eighteen electron sets and V the sum of valence electrons from the metal atoms and the ligands. The empirical formula generates the cluster k values and some of these are given in Table 2. It was also observed that the cluster such as Os6(CO)18, the k value was 12 and the number of valence electrons 84. Hence its code is M-6-12-84. Clearly V =14n and k = 2n. The same situation prevails for Os7H2(CO)20 where k = 14 and V = 98. Hence the code is M-7-14-98. In this case as in the previous complex, S = 14n = 98 and k =2n = 14. Thus, these two clusters belong to the same series. Furthermore, both these clusters are mono-capped, the former the capping is on a trigonalbipyramid geometry [M-5] while latter the capping is on an octahedral geometry. Using the 14n as a baseline, the other clusters series were derived. For example, Mn2(CO)10; the code is M-2-1-34. This corresponds to the series S = 14n+6 and k = 2n-3. For the cluster Ir4(CO)12 , k = 6 and V = 60. Hence the code is M-4-6-60 where S = 14n + 4 and k= 2n-2. The series of other clusters for the metal complexes and main group elements were derived and selected ones are given in Tables 4 and 5 and the general one is Table 3 which can be used to classify a given cluster into closo, nido, arachno and hypho or any other type.
Classification of Selected Clusters of the Main Group Elements
The examples for illustration are taken from the article on Polyhedral Skeletal Electron Theory on the website10 and the 4n rule will be applied. The first one is Pb102―; n = 10, 4n = 40 and V = 42. Hence the series is of the type S = 4n+2. This belongs to CLOSO series and k= 2n-1 = 19. Since this is a closo cluster, it will correspond to B10H102― (k= 19). The S42+ cluster; n = 4, 4n = 16 and V= 22. Thus S = 4n+6 placing the cluster into ARACHNO series with k = 2n-3 = 5. This implies the shape of a square with 5 bonds. The fifth one is probably a delocalized bond giving a shape of a regular square. The B5H54― cluster; n = 5, 4n = 20, V = 24 and hence S = 4n +4 . This cluster belongs to NIDO series with k = 2n-2 = 8. This characteristic to a square pyramid shape. The carborane cluster C2B7H13; n = 9, 4n = 36 and V = 42. Hence, S = 4n+6. This is an ARACHNO series with k =2n-3 = 15. The clusterC2B7H13 corresponds to (BH)9(H)6 = B9H15borane. The P4 molecule has n =4, 4n = 16 and V= 20. Therefore S = 4n + 4. This places the cluster into the NIDO category with k = 2n-2 = 6. This is a characteristic of a tetrahedral geometry. Its cluster code is M-4-6-20. If 10n electrons(40) are added to the valence electrons the code becomes M-4-6-60. This is the same as Ir4(CO)12 whose skeletal atoms also have got a tetrahedral shape. The P4S3 cluster; n = 7 and 4n = 28 and V= 38. Hence S = 4n+10 with k = 2n-5 = 9. This corresponds to a modification of the P4 geometry by inserting 3 S atoms into three of the six tetrahedral bonds and thus creating the 9 linkages. The last one is the hydrocarbon C6H14; n = 6, 4n = 24 and V =38. Therefore S = 4n +14 and k = 2n -7 = 5. This value gives rise to some hydrocarbons isomers some of which are presented in Scheme 6.
Conclusion
The transition metal carbonyl clusters can be classified using the 14n rule where n is the number of skeletal atoms in the cluster. The 14n is used as a basis upon which the type of cluster series of a given complex is deduced. The cluster series in Table 3 were derived using the cluster number k obtained froman empirical formula explained above using the knowledge of 18 electron rule and cluster valence electrons. The cluster number can also be calculated from the cluster series of the complex. Arising from the simplicity of the 14n rule in categorizing a cluster, a large number of clusters were analyzed. The method also revealed the existence of nuclear inner core closo clusters in some of the large clusters. For instance, the method indicated that the cluster Ni38Pt6(CO)48H5―, with a code C38C[M-6] has an octahedral nuclear cluster inner core surrounded by 38 cluster atoms in a form of capping. A cluster code notation of clapping series has thus been introduced. Furthermore, it is exceedingly fascinating to discover that in this complex the octahedral nucleus comprised of the six platinum atoms. In such clusters the central inner core acted as a nucleus around which other atoms clustered in the form of capping. However there were some clusters whose inner core nuclei could not be defined by this method. The use of 14n rule or 4n rule to categorize a given cluster of a transition metal carbonyl or main group element is easy, smooth and versatile. The empirical formula k = ½ (E-V), the 4n and 14n rules as well as the cluster series were extracted from the lurking relationship existing between the valence electrons, the number of skeletal elements and the octet and eighteen electron rules. This article highly underpins the powerful insights of Lewis’s octet rule14and Langmuir’s 18 electron rule15 of more than ninety years ago.
References
- Wade, K. ,J. Chem. Soc., Dalton, 1971, 792-793.
- Mingos, D. M. P., Nature Physical Science, 1972, 236, 99-102.
- Jemmis, E. D., J. Am. Chem. Soc., 2001, 123(18), 4313-4323.
- Jemmis, E. D., Balakrishnarajan, M. M.,Pattath, D., Chem. Rev, . 2002, 102(1), 93-144.
- King, R. B., Inorg. ChimicaActa, 1986, 116, 99-107.
- Teo, B. K., Longoni,G., Chung, F. R. K., Inorg. Chem., 1984, 23, 1257-1266.
- Zanello, P. in ‘Unusual Structures and Physical Properties’, Edited by Glein, M.,Gielen, M, Willen, R., Wrackmeyer, John Wiley and Sons, New York, 2002.
- Zanello, P., Rossi, F., PortugaliaeElectrochimicaActa, 2011, 29(5), 309-327.
- Belyakova, O., A., Slovokhotov, Y. L., Russian Chemical Bulletin, International Edition, 2003, 52(11), 1-29.
- En.wikipedia.org/wiki/Polyhedral_skeletal_electron_pair_theory, downloaded 11-05-2015.
- Kiremire, E. M., Orient. J. Chem., 2015, 31(1), 387-392.
- Kiremire, E. M., Orient. J. Chem., 2014, 30(4), 1475-1485.
- Critchley, G., Dyson, P.J., Johnson, B. F. G., McIndoe, J. S., O’Reilly, R. K., Langridge-Smith, P. R. R., Organometallics, 1999, 18, 4.090-4097.
- Langmuir, I., J. Am. Chem. Soc., 1916, 38(4), 762-785.
- Jensen, W. B., J. Chem. Educ., 2005, 82, 28-29

This work is licensed under a Creative Commons Attribution 4.0 International License.









