Carboxylate Functionalized Chitosan/Bentonite Composite Matrix as a Cation Exchanger for the Removal of Pb(II) From Aqueous Media: Kinetic and Equilibrium Studies
T. S. Anirudhan1,*, S. Rijith2, V. S. Sumi3, P. K. Anitha4, S. Abhilash5, S. M. A Shibli6
*1,5Department of Chemistry, University of Kerala, Kariavattom, Trivandrum-695 581
2Department of Chemistry, S N College, Kollam 691001,
3Department of Polymer Chemistry, Govt. College, Attingal, Thiruvanthapuram,
4Department of Chemistry, S N College, Kannur,
5Department of Chemistry, S N College, Chempazhanthy,
Curresponding author E.mail: tsani@rediffmail.com
DOI : http://dx.doi.org/10.13005/ojc/310263
Article Received on :
Article Accepted on :
Article Published : 05 Jun 2015
A novel composite matrix polymethacrylic acid-grafted Chitosan/Bentonite (PMAA-g-CS/B) was prepared through graft copolymerization reaction of methacrylic acid and chitosan in the presence of bentonite and N,N’- methylene-bisacrylamide as cross linker. The composite was well characterized using FTIR, XPS, SEM, TG/DTG, surface area analyzer and potentiometric titrations. The adsorption behavior of the composite towards Pb(II) from water and simulated battery manufacturing wastewater was studied under varying operating conditions. The kinetics of adsorption as well as adsorption isotherms at different temperatures was studied. Adsorption-desorption experiments over four cycles illustrate the feasibility of the repeated uses of this composite for the extraction of Pb(II) from aqueous solutions.
KEYWORDS:Pb(II) removal; Isotherm; Adsorption Kinetics; Desorption
Download this article as:| Copy the following to cite this article: Rijith S, Anirudhan T. S, Sumi V. S, Anitha P. K, Abhilash S, Shibli S. M. A. Design, Carboxylate Functionalized Chitosan/Bentonite Composite Matrix as a Cation Exchanger for the Removal of Pb(II) From Aqueous Media: Kinetic and Equilibrium Studies. Orient J Chem 2015;31(2). |
| Copy the following to cite this URL: Rijith S, Anirudhan T. S, Sumi V. S, Anitha P. K, Abhilash S, Shibli S. M. A. Design, Carboxylate Functionalized Chitosan/Bentonite Composite Matrix as a Cation Exchanger for the Removal of Pb(II) From Aqueous Media: Kinetic and Equilibrium Studies. Available from: http://www.orientjchem.org/?p=9071 |
Introduction
Lead(Pb) is known to be a common water pollutant with highly toxic nondegradable heavy metal and which is easily accumulated in human body (1). Even at very low concentration of Pb(II) ions, which can accumulate on humans and cause serious damage to the hematopoietic system and cardiovascular system (2,3).The bureau of Indian Standards (BIS) and world health Organization(WHO) have prescribed 50 μg/L of lead as the desirable limit for drinking water (4,5). Several methods such as chemical precipitation, electro floatation, ion exchange, reverse osmosis and adsorption have been developed for the removal of heavy metals from water and wastewater. Among these, adsorption has emerged as the front line of defense, especially for metals which cannot be removed by other techniques (6). Among them, adsorption is widely applied due to its lower cost, high efficiency, simple design, and strong operability (7).
Chitosan (CS) which contains β-1,4- linked glucosamine repeating units, is the third most naturally abundant natural biopolymer next to cellulose and chitin. Its unique properties of bio-renewability, compatibility, degradability and non toxicity. It is easily amenable to modifications, which can be achieved by chemical, physical and enzymatic methods (8). The modifications are achieved due to the presence of –OH and –NH2 backbones. The chemical modifications such as quarternization, N- alkylation, thiolation and glycations was done on the free amino moieties thereby reduce the solubility of CS (9). The use of materials with surface functional groups, such as carboxyl group shows improved selectivity for the removal of heavy metals in aqueous systems. The objective of this work is to determine the feasibility of using PMAA-g-CS/B composite matrix as an adsorbent for the removal of Pb(II) ions from water and nuclear industry wastewater. It was proposed to take advantage of the cation exchange capacity of the carboxyl group from the adsorbent surface to remove Pb(II) ions from aqueous solutions.
Materials and Methods
Analytical grade chemicals were used through out the investigation. Aqueous standard solution of Pb(II) was prepared by dissolving an accurately weighed amount of Pb(NO3)2 (Fluka) in distilled water so as to yield a metal ion concentration of 1000 mg/L. The methacrylic acid (MAA) was obtained from Fluka, Switzerland. Analytical grade potassium persulfate (KPS), and N,N‘-methylenebisacrylamide (MBA) were procured from E-Merck, India. Ltd. Chitosan was purchased from Central Institute of Fisheries Technology, Kochi, Kerala. Clay used for this work is natural bentonite clay obtained from M/S Ashapura Clay Mines. All the required working solutions are prepared by diluting the stock solution with distilled water. The concentration of Pb(II) was determined by Atomic Absorption Spectrophotometer. The amount of Pb(II) sorbed was calculated from the difference between the initial concentration and the equilibrium one.
Instrument/ Apparatus
IR spectra of the adsorbent materials were taken by using a Perkin Elmer 1800 model IR spectrophotometer. TG-DTA analysis was done by using a Metler Toledo Thermoflex instrument. pH measurements were made on a µ processor Systronic pH meter (model 361), XRD patterns of the samples were done using Siemens D 5005 X-Ray unit. SEM analyses of the samples were done using a Philips XL30 CP Hitachi Scanning electron microscope. The electrophoretic mobility measurements, µE, were carried out using a Zeta Meter system 3.0 Plus (Zeta Meter Inc., USA). Surface area was measured by low temperature nitrogen adsorption and the data were interpreted using BET equation. The nitrogen adsorption tests were carried out in a quantasorb surface area analyzer (model Qs/7) at 77 K. A temperature controlled water bath shaker (Labline shaking incubator) with a temperature variation of ± 1°C was used for equilibrium studies.
Adsorbent Preparation
CS was the starting material for the preparation of PMAA-g-CS/B composite. 5.0 g of CS in 30 mL distilled water is taken in a three neck round bottom flask, and MAA (15 ml) was added to it. The content was stirred well using magnetic stirrer for 15 minutes. 2.5 g of bentonite soaked into 15 mL water containing 0.75 g N,N’Methylenebisacrylamide and the resultant mixture kept under N2 atmosphere was then refluxed at 60 °C for 3 h using KPS as initiator .The polymer obtained after cooling was collected, and then washed with distilled water until it is free from homopolymer. The product was dried at 60 °C and then sieved using 80 – 230 mesh to get an average particle diameter of 0.096 mm. The obtained material was used for further studies.
Experimental Procedure
A stock solution of 1000 mg/L lead was prepared by dissolving appropriate quantity of Pb(NO3)2 in 1L. Batch experiments were conducted to study the extent of Pb(II) adsorption by PMAA-g-CS/B. In order to optimize Pb(II) removal conditions, the effects of pH, Contact time, initial concentration, adsorbent dosage and reusability of the adsorbent were comprehensively tested. For the batch adsorption experiment 50mL of solution containing Pb(II) ions were mixed with 0.1 g of the adsorbent at different concentrations (100-250 mg/L), temperatures (20-50 °C) and pH values (2.0-8.0) in 100 mL glass stoppered flasks. The pH of solutions was adjusted by using 0.1 M HCl or 0.1 M NaOH solutions before adsorption analysis. For the adsorption isotherm experiments, the initial solution pH was 5.5, while the initial Pb(II) concentration in the solution varied between 100 and 250 mg/L. The flasks were shaken in a thermostatic shaker for 3 h with the mixing rate of 200 rpm and the solid phase was separated by centrifugation. The amount of metal ion sorbed at time t (qt), was calculated from the mass balance equation
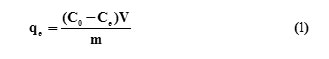
where C0 and Ce are the initial and equilibrium Pb(II) concentrations (mg/L) respectively, m is the mass of PMAA-g-CS/B (g) and V is the volume of the solution (L).
Results and Discussion
Adsorbent Characterization
The IR spectra of CS, PMAA-g-CS/B, and Pb(II)-loaded- PMAA-g-CS/B are shown in Figure 1 (A). The functional group of chitosan such as amino and hydroxyl group are very important for adsorption. The peak involved at the wave number 3500.8 cm-1 is due to the presence of –NH2 and H bonded –OH group in chitosan. The peak at 1091.7 cm-1 corresponds to the symmetric stretching of –C-O-C- stretching. After CS was cross linked with MAA, the spectrum exhibits many alternations. The wide absorption band at 3500.8 cm-1 corresponding to the stretching vibration of –NH2 and -OH group in CS shows a significant change to higher wave number. The band at 1695 cm-1 corresponds to C =O confirms the presence of MAA in the polymer composite. The peak at 1392.6 cm-1 corresponds to –C-N group. An obvious change in the peak position and intensity at 900 – 500 cm-1 region of the Pb(II)-loaded PMAA-g-CS/B spectrum could be assigned to the formation of Pb–O and O-Pb-O bond. In Pb(II) loaded PMAA-g-CS/B distinct peak at 918.1 cm-1 and changes in peak position and intensity around 550 – 1000 cm-1 can be assigned to asymmetric stretching vibration of Pb-Oand stretching vibration of weakly bonded O atom with Pb(II).
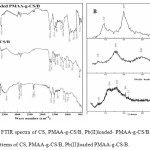 |
Figure1: (A) FTIR spectra of CS, PMAA-g-CS/B, Pb(II)loaded- PMAA-g-CS/B. (B) X-ray diffraction patterns of CS, PMAA-g-CS/B, Pb(II)loaded PMAA-g-CS/B. Click here to View figure |
The XRD patterns of CS and PMAA-g-CS/B and Pb(II)-loaded PMAA-g-CS/B are shown in the Figure 1 (B). The diffractograms of CS, PMAA-g-CS/B, Pb(II)-loaded- PMAA-g-CS/B are compared to determine the change in crystallinity.
In CS, XRD pattern, the two strong peaks in the diffractogram of chitosan at 2θ at 9.34°, 19.80°, their crystal lattice constant corresponding to 8.470 and 4.075. The peak at 2θ about 19.8° is attributed to the allomorphic tendon form of CS, which resulted in a strong decrease in sorption capacities (10). However, the peak found at 9.34°, in the diffractograms of PMAA-g-CS/B was disappeared, this indicates that polymerization decreases the crystallinity. The intensity of peaks on the diffraction pattern of PMAA-g-CS/B also indicates that the crystallinity decreases after functionalization, 2θ = 16.48°. The change in d spacing and width shows that there is a substantial decrease in crystallinity of CS on modification. In general, modified CS decreases its crystallinity compared with original CS. After Pb(II) loading onto PMAA-g-CS/B new broad peaks at 6.02°, 12.16° and 16 .32° was due to the decrease in space between the polymer lattices after Pb(II) sorption. This also indicates the involvement of active surface moieties for Pb(II) adsorption.
The surface structural features of the CS, PMAA-g-CS/B and Pb(II) loaded PMAA-g-CS/B were studied using SEM. The micrographs obtained for these materials are shown in Figure 2(A). The surface of CS is non-particulate and abundant in folds. The PMAA-g-CS/B polymer composite displays a more extensive unfolded 3D network; it is attributed to the cross linking reaction leading to the formation of closely packed chain rearrangement. The SEM pictures of polymer composite verify that graft polymers have a porous structure. It is supposed to that these pores have the region of water penetration and interaction site of external stimuli with the hydrophilic groups of the graft polymers. A SEM picture also shows that there is a crack on the surface which confirms the miscibility of the prepared polymer composite. When polymer composite is loaded with Pb(II) it shows a highly porous film structure. After Pb(II) adsorption, the SEM image shows the uniform distribution of Pb(II) ions on the surface of PMAA-g-CS/B. So we can conclude that the adsorption experiments are highly effective.
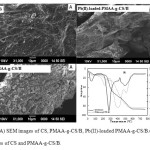 |
Figure2: (A) SEM images of CS, PMAA-g-CS/B, Pb(II)-loaded PMAA-g-CS/B.(B) TG and DTG curves of CS and PMAA-g-CS/B. Click here to View figure |
The TG/DTG curves for CS and PMAA-g-CS/B are shown in Figure 2(B). The first set of decomposition was at 80 – 100 °C region, with a mass loss of 5-8 % is due to the loss of water that was physically adsorbed on the material surface. The second stage reached a maximum of 294 °C for CS and 230 °C for PMAA-g-CS/B. It is having the mass loss of 34.7 and 24.3 %, respectively. The third stage decomposition for CS and PMAA-g-CS/B are in the range of 310-650 °C having a mass loss of 92.0 and 85.0 % respectively. From the DTG curve it is noted that chitosan shows three decomposition stages with maximum temperatures at 95, 294 and 498 °C and PMAA-g-CS/B shows maximum temperatures at 247, 403 and 530 °C. This decomposition pattern indicates that thermal stability of PMAA-g-CS/B is much better than CS.
Density of the adsorbents was calculated by Picnometric method in which water was used as a displacing liquid. The density of CS and PMAA-g-CS/B was found to be 0.8 and 1.33 g/mL, respectively, and the values of Cation Exchange Capacity (CEC) of CS and PMAA-g-CS/B were found to be 1.54, and 2.24 meq/g, respectively. The surface area of CS and PMAA-g-CS/B was determined using BET N2 adsorption method and was found to be 13.4 and 29.7 m2/g, respectively.
Adsorption Experiments
Effect of pH
The initial pH value of the solution has a significant effect on the adsorptive uptake of metal ions presumably due to its impact on the surface binding-sites and also the surface charge of the adsorbent. The results of the effect of pH in the range of 2.0 – 8.0 on the removal extent of Pb(II) are summarized in figure 4(A). At low ph conditions, H+ cations compete with Pb(II) cations and occupy the exchange sites on PMAA-g-CS/B. The surface charge of PMAA-g-CS/B was also illustrated in Fig 4(B). In Fig. 4(B), pHZPC for PMAA-g-CS/B is about4.3. At pH< ζ , the surface of the sorbent is positively charged , while it is negatively charged at pH> ζ. Hence repulsion between Pb(II) species and positively charged surface at stronger acidic conditions. Hence, increase the pH, this repulsive interaction decrease and the removal of Pb(II) also increase. This phenomenon was happened by increasing the pH from 1 to 4.5 and then the deviation is negligible to pH 5.0. At this pH lesser number of H+ ions are present in the medium and also lesser number o surface adsorption site will be protonated, and can be remove more Pb(II) cations from solution. An undesirable sharp increasing trend was observed at the pH range of 5.0-6.0, which was attributed the precipitation of Pb(II) cations starts from a pH of 5.5. To eliminate this effect, the extent of Pb(II) precipitation was studied with respect to blank solutions without PMAA-g-CS/B at each pH. After the pH of 5.5 significant precipitation was observed which illustrate the negative impact on the removal of Pb(II) by ion exchange processes thus the removal of Pb(II) with pH was optimized at a maximum pH of 5.5. As increase in pH the competition between hydroxyl anion and Pb(II) towards adsorption sites and adsorption of Pb(II) ions concentration was declined and concentration of OH– increases.
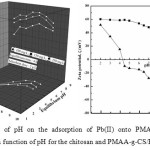 |
Figure4: (A).Effect of pH on the adsorption of Pb(II) onto PMAA-g-CS/B. (B) Zeta potential, ζ as a function of pH for the chitosan and PMAA-g-CS/B. Click here to View figure |
Adsorption Isotherm
The results of the Pb(II) adsorption isotherm experiments are shown in Figure 5(A). The adsorption capacity considerably increased with the Pb(II) equilibrium concentration and temperature. The adsorption isotherms of PMAA-g-CS/B were analyzed according to non-linear forms of the following isotherm models:
Langmuir: qe = Q0bCe/ 1+bCe ; Freundlich: qe = KFCe1/n ; Redlich–Peterson model: qe = KRCe / 1+bCeβ ; Where Ce is the equilibrium concentration of Pb(II) on adsorption in mg/L and qe is the amount adsorbed at equilibrium in mg/g. Qo and b are the Langmuir constants related to adsorption capacity and energy of adsorption, respectively. KF and 1/n are Freundlich constants related to adsorption capacity and intensity of the adsorption respectively. KR, β and b are Redlich-peterson isotherm constants related to adsorption capacity, empirical exponent and energy of adsorption, respectively. The adsorption capacity Qo increases with increase in temperature. It has increased from 99.9 to 126.6 mg/g for Pb(II) adsorption with rise in temperature from 20 to 50 oC. The value of b increased from 0.24 L/mg to 0.46 L/mg with rise in temperature from 20 to 50 oC. This indicates that adsorption rate increases with increase in temperature. It should be noted that β normally lying between 0 and 1, indicated a favorable adsorption for the studied adsorbent. Among these adsorption models, the best correlations were observed for Langmuir model.
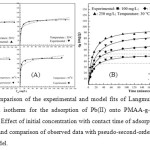 |
Figure5:(A). Comparison of the experimental and model fits of Langmuir, Freundlich and Redlich-Peterson isotherm for the adsorption of Pb(II) onto PMAA-g-CS/B at different temperatures.(B) Effect of initial concentration with contact time of adsorption of Pb(II) onto PMAA-g-CS/B and comparison of observed data with pseudo-second-order and pseudo-first-order kinetic model. Click here to View figure |
The values of R2 and c2 obtained for the adsorption of Pb(II) on PMAA-g-CS/B follow Langmuir adsorption isotherm. The fact that the Langmuir isotherm fits the experimental data very well may be due to homogeneous distribution of active sites on PMAA-g-CS/B, since the Langmuir equation assumes that the surface is homogeneous.
Adsorption Kinetics
Batch kinetic experiments were performed to determine the time required for Pb(II) adsorption process to reach equilibrium kinetic results are presented in Figure 5(B). It can be seen that Pb(II) adsorption onto PMAA-g-CS/B composite reached equilibrium after a certain time of ~180 min, yielding a maximum adsorption of 51.5, 72.9, 82.3 and 98.7 mg/g at an initial concentration of 100, 150, 200 and 250 mg/L, respectively. Based on these results, a contact time of 3 h was selected for all subsequent batch experiments. Pb(II) uptake increases with the increase in initial Pb(II) concentration. This is due to the presence of more number of Pb(II) ions in the solution. To interpret the experimental data properly, it is necessary to determine the steps in the adsorption process, governing the overall removal rate for the system. Also, the rate-limiting step is important in the field application point of view. The rate-limiting step of the adsorption process can be calculated using the pseudo-first-order and pseudo-second-order kinetic equations (11).
The non linear kinetic equations may be written as
pseudo-first-order: qt = qe (1-e-k1t) ; pseudo-second-order : qe = k2 qe2 t / 1+K2qet
Where qe and qt are the amounts of solute adsorbed per unit mass of the adsorbent at equilibrium and time t, respectively. k1 and k2 are the pseudo-first-order and pseudo-second order rate constants. The values of k1 and k2 can be obtained from non linear regression analysis. The values of k1 were found to be increased from 9.1×10-2 to 16.2 × 10-2 min-1 while pseudo-second-order rate constants were decreased from 1.43×10-3 to 0.54×10-3 g/mg/min for initial Pb(II) concentration of 100, 150, 200 and 250 mg/L, respectively. It was observed that the value of k2 decreased with increased initial concentration. The calculated qe values agree very well with the experimental values and the values of regression coefficient of the above show that the model can be applied for the entire adsorption process and confirms the chemisorption of Pb(II) onto PMAA-g-CS/B. The values of χ2 arealso considerably less for pseudo-second-order kinetic model reinforcing the applicability of pseudo-second-order kinetic model.
Effect of adsorbent dose with simulated battery manufacturing wastewater
Batch equilibrium experiments were performed to test the effectiveness of PMAA-g-CS/B composite to recover Pb(II) in simulated battery manufacturing wastewater sample. Pb(II) containing (100 mg/L) simulated battery manufacturing wastewater sample having the composition Pb(II) 5.0 mg/L), Ca(II) 60 mg/L, Mg(II) 15 mg/L, Na(I) 20 mg/L, K(I) 3 mg/L, SO42- 170 mg/L, Cl– 30 mg/L, NO3– 3 mg/L, SiO2 8 mg/L, pH 5.0 was used (12). The effect of adsorbent dose on Pb(II) removal from simulated battery manufacturing wastewater sample was studied at different adsorbent doses ranging between 0.25 and 10.0 g/L using chitosan, Bentonite, PMAA-g-CS/B and Amberlite IRC-50. The percentage removal increases with increase of adsorbent dose. For the quantitative removal of 100 mg/L Pb(II) from 1,0 L simulated battery manufacturing wastewater, a minimum adsorbent dosage of 8.2 g chitosan or 7.0 g bentonite was used. The dosage of 2.5 g Amberlite IRC-50 or 2.0 g PMAA-g-CS/B was required for the complete removal of Pb(II) from simulated battery manufacturing wastewater. The results clearly show that PMAA-g-CS/B is about 4.0, 3.75 and 0.75 times more effective than CS and, Bentonite respectively and it shows enhanced performance as compared to synthetic ion exchanger like Amberlite IRC-50.
Desorption and Regeneration Studies
Desorption tests were carried out using different types of 0.1 M desorbing agents such as NaOH, Na2SO4, NaCl, NaNO3, HCl and HNO3. The percentage desorption for NaOH, Na2SO4, NaCl, NaNO3, HCl and HNO3 was 56.3, 61.4, 62.9, 66.3, 82.1 and 97.2 %, respectively. Among these, 0.1 M HNO3 was proved to be the most suitable desorbing reagent. After four cycles, the adsorption capacity of the PMAA-g-CS/B decreased from 99.8% to 90.1%. And the recovery of Pb(II) ions in 0.1 M HNO3 decreased from 97.2 % in the first cycle to 81.0 % in the fourth cycle. Adsorption-desorption operations illustrating stabilized performance of the sorbent in repeated cycles.
Conclusions
In the present study, a novel adsorbent, PMAA-g-CS/B composite was prepared and characterized. Its efficiency in removing Pb(II) was tested by batch adsorption technique. The pH 5.5 was found to be optimum for the adsorption of Pb(II) on PMAA-g-CS/B. Upon various models applied the pseudo-second-order kinetic model and Langmuir isotherm model gave good correlation with the experimental data. Attempts for quantitative removal of Pb(II) from simulated battery manufacturing wastewater using variable adsorbent doses were employed and satisfactory results were obtained. Repeated adsorption-desorption study showed that PMAA-g-CS/B can be effectively used as an adsorbent for the removal and recovery of Pb(II) from aqueous solutions.
Acknowledgements
The authors are thankful to the Head, Department of Chemistry, University of Kerala, Trivandrum for providing laboratory facilities. Our wishes also reflect our deepest recognition to those who encouraged us to finish this work by signs of friendship which we are grateful.
References
- Sultan bayeva, G.; Holze, R.; Chernyakova, R.M.; Jussipbekov, U. Micropor. Mesopor. Mater. 2013, 170, 173-180.
- Patrik, L. Alternat. Med. Rev. 2006, 114-127.
- Torres-Blancas, T.; Roa-Morales, G.; Fall, C.; Barrea-Diaz, C.; Urena-Nunez, F.; Pavon Silva, T.B. Fuel. 2013, 110, -11.
- BIS, 2012 Second Revision IS: 10500:2012, Bureau of Indian Standards, New Delhi, India.
- WHO 1984 Guide Lines for Drinking-Water Quality, Volume 2, Health Criteria and other Supporting Information. World Health Organization, Geneva.
- Mohan. D.; Pittman Jr, C.U. J. Hazard. Mater. 2007, 142, 1-89.
- Naushad, M.; Chem. Eng. J. 2014, 235, 100-108.
- Mourya, V.; Inamdar, N.N. React. Funct. Polym.2008, 68, 1013-1051.
- Wydro, P.; Krajeska, B.; Wydro, K.H. Biomacromolecules, 2007, 8, 2611-2617.
- Chassary, P.; Vincent, T.; Guibal; E. React. Funct. Polym. 2004, 60, 137-149.
- Anirudhan, T.S.; Rijith S.; Divya, L. Sep. Sci. Technol. 2009, 44, 2774-2796,
- Noeline, B.F.; Manohar, D.M.; Anirudhan, T.S. Sep. Purif. Technol. 2005, 45, 131–140

This work is licensed under a Creative Commons Attribution 4.0 International License.









