Acid-Promoted Hydrolysis of m-Cl-Phenyl Phosphorotriamidate leading to its Highly Basic Nature by Kinetic Means
Harish Kumar Amb* and Shashi Prabha
Department of Chemistry, Govt. P.G. College, Guna (M.P.) India. harishkumar.hk559@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/310220
Article Received on :
Article Accepted on :
Article Published : 01 Jun 2015
Hydrolysis of m-Cl-Phenyl Phosphorotriamidate has been performed in the acid range, 0.01 to 7.0 M –HCl,in 12%AcoH-H2O (v/v) at 98(±0.5)oC. The continuous second-order rate rise with the absence of a rate maximum in the entire acid range is significant, leading to its highly basic nature. In this respect, it differs from other related(o-Cl-Ph-&p-Cl-Ph-) members of the phosphorotriamidate group. The salt effect variable study leads to the presence and reactivity of the two major reactive species with the Neutral Species(I) working in the entire acid range; while the Conjugate acid form(II) was observed operating between 4.0-7.0 M HCl region only. Both uni-and bi-molecular mechanisms for the two reactive forms with P-N bond fission have been decided for the C-N-P ester. The hydrolysis is shown to be decreased by the action of the series of the nucleophilic reagents and I'shows the optimum effect here. Role of a chloro-group in the unusual meta position in each aryl matix during hydrolysis was particularly the important feature of this study.
KEYWORDS:Hydrolysis; Neutral Species; Conjugate acid species; Basicity
Download this article as:| Copy the following to cite this article: Amb H. K, Prabha S. Acid-Promoted Hydrolysis of m-Cl-Phenyl Phosphorotriamidate leading to its Highly Basic Nature by Kinetic Means. Orient J Chem 2015;31(2). |
| Copy the following to cite this URL: Amb H. K, Prabha S. Acid-Promoted Hydrolysis of m-Cl-Phenyl Phosphorotriamidate leading to its Highly Basic Nature by Kinetic Means. Available from: http://www.orientjchem.org/?p=8952 |
Introduction
Organic phosphoramidate have been widely utilized for synthetic1,2 work, especially for the synthesis of pyrophosphates. These compouds may also function as fireproof materials3 (e.g. phosphoric aniline diamide); as polymers 4,5 and co-polymers (Aromatic araliphatic compounds based on Phosphoric acid and Thiphosphoric acid amides). The latter are then suitable for making films, membranes and fibers etc. Organic phosphoramidates even have the potential for their function as the harmful insecticides,6 while others may act as very useful drugs for the treatment7 of AIDS and Cancer etc.
Experimental
The m-Cl-Phenyl Phosphorotriamidate had been synthesized by refluxing for ca.8 hrs. with morpholine as catalyst. The ratio of reactants such as m-Cl-Aniline,POCl3 and Morpholine was 3:1:3 in a dry benzene medium. A creamish-solid was obtained after total refluxing period and this solid after treatment with CHCl3, H2O and acetone, a white substance with yellowish tinge was formed, having a sharp m.p.251-253oC. Its purity was checked with tlc, and the single sharp spot was observed as desired. It was confirmed as the m-Cl-phenyl phosphorotriamidate on the basis of quantitative P content estimation.
Kinetic study of acid-catalysed hydrolysis(98±0.5)oC of m-Cl-Phenyl phosphorotriamidate was made using Allen’s modified method. During the kinetic study the optical density was measured with help of Systronics-118: UV-Vis. spectrophotometer. Concentration of the compound was maintained as 8.0×10-4 M throughout the study, except for concentration effect study.
Results and Discussion
m-Cl-Phenyl Phosphorotriamidate, a C-N-P bearing ester, has been synthesised in this chemical laboratory and examined for its hydrolysis in 12%AcOH-H2O medium, in the acid range 0.01 to 7.0 M-HCl, at 98(±0.5)oC.The estimated second-order rate coefficients have been presented in Table I, showing their general trend, in the entire acid region examined. Allen’s modified method8 has been used to quantitatively determine the extent of hydrolysis on the basis of phosphorus liberated as H3PO4 component. At 4.0 M-HCl and onwards, the observed rate coefficients rise continuously till 7.0 M-HCl under the common set of reaction conditions. The acid-rate profile (not shown) showed that there is a plateau in the acid range, 0.3 M to 3.0 M-HCl. After this, the rates show a steep rise without involving any rate maximum in this higher zone of acid concentrations. This pattern of rates reflects the kinetic behaviour of strongly basic compounds, the amides9,10 in this molecule as well.
In order to determine the presence as well as the contribution of the reactive forms, the Neutral electrolyte effect study11 had been made keeping the ionic strengths (µ) as 1.0, 2.0 and 3.0, using suitable compositions of both LiCl and conc. HCl. In Fig.1, three linear curves are seen to be formed, with different slopes (KH+) but making a common intercept (KN) on the Y-axis. Using the IInd empirical term12 of Debye Hückel equation correlation(і) is used to calculate the total rates for the contributory reactive species :
 |
figure 1 Click here to View figure |
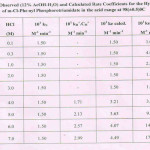 |
Table 1 Click here to View table |
Thus, ke kN kH+.CH+ ……(і)
Also, kN = kNo × ebNµ ………..(іі)
Equation (іі) is simplified, as Fig.1 shows that the Neutral species (I) makes a fixed contribution, so that:
kN = kNo =1.50×10-1 M-1min-1 ………….(ііі)
Further : kH+.CH+ = kHo+.CH+.ebH+.µ ………(іv)
where, kH+, CH+ and kHo+ represents the acid-catalysed rates via the Conjugate acid species(II), the concentration of the H+ -ions and the rates via the Conjugate acid species(II) at zero ionic strength only.
Logarithmically, equation (іv) is represented as :
log kH+.CH+ = log kHo+. log CH+ +.b’ H+.µ …………(v)
It may be seen that the Neutral species (I) makes a constant contribution in the acid region, particularly between 0.01 to 3.0 M-HCl (Table I). The acid-catalysed rate (kH+) at zero ionic strength (1+ log kHo+ =0.63) is obtained (Fig.not shown), while b’H+ =0.00. Using all these parameters in equation (v), the rates via Conjugate acid species(II) were determined at each acid molarity separately. Table I includes this data, and it may be noticed that there is a similarity between the calculated and the observed rate coefficients via the Neutral form(I) in 0.1-3.0 M-HCl region only. Between 4.0 M to 7.0 M-HCl, both the Neutral and the Conjugate acid (II) species are both found to contribute toward the overall rates of hydrolysis. However, the differences between ke observed and ke calculated values are due to the exceptional basic character shown by the m-Cl-phenyl phosphorotriamidate under observation. This particular feature makes it significant for the kinetic study in particular.
In higher acid media(1.0 M-HCl and above) Hammett11 plot(slope=0.53) failed to give any concrete result. Normal Bunnett correlations13 (Figs. not included) giving w = +3.0 and w* = 1.90 are not satisfactory (see later) in this case. Bunnett and Ölsen plot13 drawn between (logke +Ho) versus [(log CH+ + Ho)] gives a slope, Φ, as + 0.68. This favours the role of water both as a nucleophilic as well as a proton –transferring agent. The latest correlation14 till date in strong acid media, the Yates and McClelland plot(based on modified-Hammett plot : m= +0.285 and–log aH2O data) gives slope, r =3.03. Both ‘r’ and ‘w’ giving identical (3.0) values strongly favour the participation of minimum three water molecules during the hydrolysis.
A simple but specific correlation (rates versus acid molarities) suggested by Bunnett for highly basic compounds was drawn (Fig. not included) giving a new slope, w¢ = 4.69. This value suggests that water acts as a second reactant, in the rate-determining step of the present hydrolysis. The slope of the special Bunnett plot thus leads to the role of around four to five water molecules, during the hydrolytic reaction. This value strongly supports the recently identified minimum sized-but stable hexahydrated-proton species15, [H+(H2O)6] to bring about protonation, in spite of having a very short span of life.
The Arrhenius parameters (Table II) were determined from rate data determined at three different (98, 90 & 80oC) temperatures at 0.1 M-HCl in 12%AcOH-H2O medium. The well-known Arrhenius equation was thus used leading to the suitable Arrhenius parameters. The high energy of activation11, Ea and the high collision factor (A) support respectively, a uni- and a bi-molecular route of hydrolysis in this case. The entropy of activation (∆S≠) being a +ve quantity (Table II) also supports the unimolecular mode of hydrolysis. Both ∆H≠ and ∆G≠ have been observed to be higher and positive favouring hydrolysis of the triamidate at the boiling water temperature i.e.at 98(±0.5)oC.
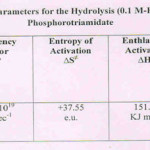 |
Table 2 Click here to View table |
Effect of change of medium from 12% to 79% AcOH-H2O medium at
2.0 M-HCl shows an increase in rates from 1.74×10-1 M-1 min-1 to 10.27×10-1M-1 min-1 favouring a transition state with charges created in it, possible only via the Neutral species(I) of the phosphorotriamidate under observation. The decrease of e of the medium with rise in AcOH % age favours protonation of the substrate i.e. the phosphorotriamidate itself.
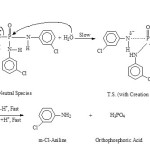 |
Scheme 1 Click here to View scheme |
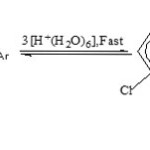 |
Scheme 2 Click here to View scheme |
Concentration effect study at 3.0 M-HCl favours second-order for acid hydrolysis. The second reactant, H2O, being in much large excess, the study suggests the overall hydrolysis belongs to the pseudo-first order type, however. They also indicate the compound to undergo direct scission. This mainly applies to the Conjugate acid species (II) of the phosphorotriamidate mainly.
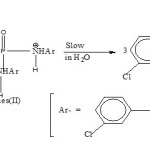 |
Scheme 3 Click here to View scheme |
Parent amine effect study at 1.0 M-HCl in 12%AcOH-H2O medium only slightly affects the rates of hydrolysis, so that during the progress of hydrolysis when it is being liberated its role as a nucleophile is also not very significant. The effect of other nucleophilic reagents(Cl’, Br’, etc.) at 4.0M-HCl (12% AcOH-H2O) at 98oC, gave the following order of reactivity:
Nucleophilic reagents: I’ < Cl’ < F’ < Br’ < H2Ö
101ke M-1 min-1 : 0.52 1.10 1.80 1.98 3.55
All the above nucleophilic reagents (0.1 M) result in a decrease in the rates of hydrolysis and the iodide ions show the least reactivity towards the phosphorotriamidate.
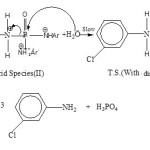 |
Scheme 4 Click here to View scheme |
The nature of the P-N bond fission was confirmed by applying an azo-dye16 test. During the progress of acid hydrolysis, the parent amine being released gave a +ve test suggestsing P-N bond fission to occur. Other comparative kinetic and thermodynamic data (not included) for related C-N-P members also supported P-N bond fission only. The progress of hydrolysis of the phosphorotriamidate is based on its highest rates shown in the form of comparative kinetic data of the total series as under :
Monoamidate Diamidate Triamidate
Compounds 1(m-Cl-Ph-NH-) 2(m-Cl-Ph-NH-) 3(m-Cl-Ph-NH-)
102 M-1 min-1 20.43 27.78 35.50
Medium 20% AcOH-H2O 10%AcOH-H2O 12%AcOH-H2O
The above comparison at 4.0 M-HCl shows that the triamidate does not hydrolyse via the formation of either the Mono- or the Di-amidate of the corresponding type. The hydrolysis thus, involves simultaneous scission of all the three subsd.-aryl units of the phosphorotriamidate, under observation.
Conclusion
Kinetic study performed during hydrolysis shows that m-Cl-Phenyl phosphorotriamidate undergoes hydrolysis via two reactive forms, Neutral Species (Bimolecular) and Conjugate acid (Uni- and Bi- molecular) with P-N bond fission, as shown below :
Mechanism (1): SN2(P) : Bimolecular hydrolysis of m-Cl- phenyl phosphorotriamidate via the Neutral Species(I) in the entire (0.01 – 7.0 M-HCl) acid region with P-N bond fission:
Mechanism(2) : SN1(P) : Unimolecular mechanism of hydrolysis via the Conjugate acid Species (II) between 4.0-7.0 M-HCl
(a) Formation of Conjugate acid Species(II) by a fast pre- equilibrium proton transfer step
(b) Simultaneous cleavage of all the three protonated units in the AcOH-H2O (12%) medium :
Mechanism (3): SN2 (P) : Bimolecular hydrolysis via the Conjugate acid Species (II) between 4.0-7.0 M, HCl :
(a)’ Formation of Conjugate acid species(II) of m-Cl-Phenyl Phosphorotriamidate: Same as given in 2(a) earlier
(b) ‘
Acknowledgement
The author, Harish Kumar Amb is thankful to Dr. Shashi Prabha, Retd. Professor & former Head, School of Studies in Chemistry, Jiwaji University, Gwalior for both guidance and providing all facilities.
References
- (a) Clark, V.M.; Kirby, G. W; and Todd ,A., J.Chem.Soc., 1957,1497 (b) Chambers, R.W.; Shapiro, P. and Kurkov V., J. Am. Chem. Soc., 1960, 82, 970
- (a) Toy,D.F.Arthur and Eilers, L. Kenneth, U.S. Patent 3937765, (2009); (b) Shejwalkar,Pushker; Nigam, P.; Rath and Bauer, B.Eike, Molecules, 2010, 15, 2631- 2650 ;(c) Delley, J.Richard, O’Donoghue, C. Ann Marie and Hodgson, R.W. David, J.Org.Chem., 2012,77 ,5829-5831 ; (d) Helge Klare, J örg M. Neudorfl and Beilstein, Bernd Goldfuss, J. Org. Chem., 2014, 10, 224-236.
- Kobayashi Etsuro, Bull. of the Chem. Soc. of Japan, 1973, 46,183-186
- Kobayashi, E.; Zasshi, Kogyo Kagaku, 1971,74, 1780
- (a) Menahem A.Kraus, Moshe A.Frommer, Mara Nemas and Rodika Gutman, U.S. Patent (19); 4, 233, 434; Nov.11, (1980); (b) James L. Bertram and William Davis, U.S. Patent (19); 4, 481, 347; Nov.6 (1984).
- Carr,Russell L.; Dail,Mary Beth, Chambers, Howard W. and Chambers ,Janice E., J. Toxicol., Hindawi Publ. Corpn., Volume 2015, Article ID 470189, 5 pages.
- (a) Cohen, M. and Westhimer, F.M., J. Am. Chem. Soc., 1952, 74,4387 (b) Moriguchi, T.; Asai, N.; Okada, K.; Scio, K.; Sasaki ,T. and Sekine, M., J. Org. Chem., 2002, 67, 3290
- Allen,R.J.L., Biochem. J., 1940,34, 858
- Edward, J.T. and Meacock, S.C.R, J. Chem. Soc., 1957,2000
- Rosenthal, D. and Taylor, T.I., J. Am. Chem. Soc., 1957, 79, 2684
- Laidler K.J, Chemical Kinetics, Third ed., Harper & Row Publishers, New York, pp.2, 24-25, 183, 202-203 (1987).
- Bailley, M.C., Bull. Soc. Chim. Fr., 1942, 340-405
- Bunnett, J.F., J. Am. Chem. Soc., 1961, 83, 2686
- Yates, K. and McClelland, R.A., J. Am. Chem. Soc., 1967,89, 2686
- Jiang,J.C.; Wang,Y.S.; Chang,H.C.; Lin,S.H.; Lee,Y.T.; Schatteburg,G. Niedner, J.Am.Chem.Soc., 2000,122, 1398-1410
- Furniss B.S, Hannaford A.J., Smith P.W.G. and Tatchell A.R., Vogel’s T.B. of Practical Organic Chemistry, Fifth ed., Addison-Wesley Longman Ltd., England, pp.166-168,199-215 (1998).

This work is licensed under a Creative Commons Attribution 4.0 International License.









