Synthesis, Characterization and Antimicrobial activity of protected dipeptides and their deprotected analogs
Jatinder Pal Kaur Gill1, Simranjeet Singh2, and Nidhi Sethi*1
1Department of Chemistry, Lovely Professional University, Phagwara, Punjab, India- 144411 2Department of Biotechnology, Lovely Professional University, Phagwara, Punjab, India- 144411
DOI : http://dx.doi.org/10.13005/ojc/310149
Article Received on :
Article Accepted on :
Article Published : 14 Feb 2015
Peptides are the chemical compounds which consist of amino acids coupled together by peptide linkage. Peptide derivatives are synthesized by coupling the carboxyl group of one amino acid with amino group of other. Due to the possibilities of fortuitous and unintentional reactions, various protecting groups are used to protect the carboxylic acid as well as amino groups of both the amino acids. These peptide derivatives are associated with a variety of pharmacological activities including antibacterial and antifungal activities. While doing our analysis some of the dipeptides were synthesized in a reasonable yield and purity which were fully characterised by FTIR and H1 NMR. The antimicrobial activity of these derivatives was studied and these were found to be active against two strains of fungi (Aspergillus fumigatus & Pencillium chrysogenum) and two strains of bacteria (E. coli and Salmonella typhimurium). This provides for a future insight to work on the synthesisof these dipeptide derivatives to achieve their stability.
KEYWORDS:Peptides; Synthesis; Characterization
Download this article as:| Copy the following to cite this article: Gill J. P. K, Singh S, Nidhi Sethi N. Synthesis, Characterization and Antimicrobial activity of protected dipeptides and their deprotected analogs. Orient J Chem 2015;31(1). |
| Copy the following to cite this URL: Gill J. P. K, Singh S, Nidhi Sethi N. Synthesis, Characterization and Antimicrobial activity of protected dipeptides and their deprotected analogs. Orient J Chem 2015;31(1). Available from: http://www.orientjchem.org/?p=7228 |
Introduction
Peptides are the constituents of proteins. They are biologically active chemical compounds formed by amino acid units linked together by amide linkage. They are useful pharmaceutical agents which are used for the treatment of arthritis,diabetes, cardiovascular diseases,immune diseases, growth problems and many more.Peptides are smaller in size, less immunogenic, highly active and more stable at room temperature than proteins and antibodies. Also they have low manufacturing cost.
Peptides are widely used as pharmaceutical agents due to the diversity of their constituents and their minimal toxicity. The 20 naturally occurring amino acids found in proteins offer a tremendous number of possible combinations for a peptide sequence1. Structural modifications of synthetic peptides also provide diversity in their pharmaceutical design. Cyclic peptides offers enhanced stability and retain biological activity2. We have described a simple three step procedure for the synthesis of dipeptides from component amino acids and checked their antimicrobial activity.
Materials and Method
Materials
BOC- protected amino acid and Thionyl Chloride used for synthesis of dipeptides were obtained from Qualikems fine chemicals private limited (New Delhi). Amino acids like L-Tyrosine, L-Leucine, L-Proline,and solvents like Dichloromethane (DCM), Methanol, Diethylether, Tetrahydrofuran (THF) and other reagents like N-Methylmorpholine (NMM), Dimethyl for mamide (DMF), Trifluoroacetic acid (TFA) were purchased from Loba Chemie Pvt Ltd (Mumbai). Coupling reagent Isobutylchloroformate (IBCF) was obtained from Spectrochem Pvt Ltd (Mumbai) and Luria Bertani (LB) agar was taken from Himedia Laboratories Pvt Ltd (Mumbai). The fungal species like Aspergillus fumigatus (NCIM-902),Pencillium chrysogenum (NCIM-738) and bacterial species; E.Coli (NCIM- 2563) and Salmonella typhimurium (NCIM-2501) used in the process are the products of NCIM, National Chemical Laboratory (Pune).
Scheme
Step1. Carboxyl group protection of L-amino acids with methyl ester
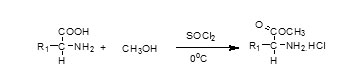
(Where amino acids taken are Tyrosine, Leucine, and Proline)
Step2. Coupling of protected amino acids to form protected dipeptide:
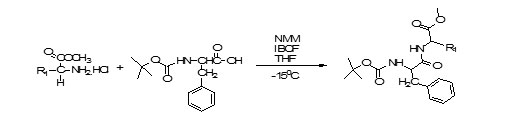
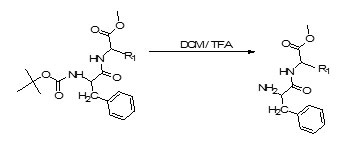
Step3. Deprotection of protected dipeptides:
Method
Protection of Carboxyl Group of L-Amino Acids Using Methanol and Thionyl Chloride3:
50 ml Methanol was taken in 250 mL round bottom flask under the ice cold conditions followed by the addition of 24 mmole of Thionyl chloridein a drop wise manner along with continuous stirring. After that 12 mmole of L-amino acid (Tyrosine, Leucine and Proline)was added pinch wise. The contents of the flask were then refluxed for 4 hours. The progress of reaction was monitored time and again by TLC on readymade silica plates (Merck, UV active max254nm) and the solvent was evaporated under reduced pressure. The residue was obtained as hydrochloride salt and was dried in vacuum desiccator using P2O5.
Coupling of Methyl Ester Protected L-Amino Acid with BOC-Protected L-Amino Acids4,5
7.5 mmole of BOC-Phenylalanine was dissolved in 20 mL of Tetrahydrofuran in a round bottom flask followed by the addition of 7.6mmole of N-Methylmorpholine. At the same time, 9mmole of methyl ester of amino acid (L- Leucine, L-Prolineand L-Tyrosine) was dissolved in minimum amount of Dimethylformamidein another round bottom flask and 9.2 mmole of N- Methylmorpholine was also added to it so as to neutralise the salt under ice cold conditions. Coupling reagent Isobutylchloroformate was then added at -15oC to first flask with vigorous stirring and contents of second flask were added to that. The reaction mixture was allowed to stir for 2 hours. The filtrate was evaporated under reduced pressure and the oily residue left was dissolved in ethyl acetate. It was then washed with 10% aqueous sodium bicarbonate, citric acid solution and finally with brine. The organic layer was dried over anhydrous sodium sulphate and evaporated under reduced pressure. Residue was then dried in vacuum desiccator using P2O5. The crude product was purified over silica gel column using Methanol : Chloroform as solvent system to get pure compound.
Deprotection of Protected Dipeptides6, 7:
BOC-Protected dipeptides were dissolved in Dichloromethane in a round bottom flask having a guard tube attached to it. About 1mL of Trifluoroacetic acid was added in the flask. The contents of the flask were allowed to stand for 1 hour. Solvent was then evaporated under reduced pressure and the oily residue obtained was triturated in diethylether to get solid residue which was then stored in desiccator.
Antimicrobial Activity
The antimicrobial activity of protected and deprotected dipeptides was checked in vitro by Kirby-Bauer disc diffusion method8. In this method the antimicrobial activity of test compounds was examined by measuring the diameter of zone of inhibition.9The antimicrobial activity was checked against two fungal strainsAspergillus fumigatus(NCIM-902), Pencillium chrysogenum(NCIM-738) and two Gram-negativeE.Coli(NCIM-2563), Salmonella typhimurium(NCIM-2501) bacterial strains.LB plates were streaked by spread plate method and discs of 1mm Whatsmann filter paper were soaked in 250 ppm, 500 ppm, and 1000 ppm of dipeptides (both protected and deprotected), along with Ciprofloxacin(1µg/disc) as a standard and solvent solution as control and placed in the centre of the inoculated petriplates. The plates were incubated at 37oC and results were interpreted after 24 hrs10.The results of antimicrobial activity of protected and deprotected dipeptides are shown in table 1 and 2.
Results and Discussion
1H NMR; 400MHz CDCl3( δppm);
1. BOC-PheLeu-OMe:0.874-0.915(m,9H,CH2CH(CH3)2), 1.33(s,9H,(CH3)3CO),1.49-1.57 (m,2H,NHCH2CH2CH(CH3)3),2.77-2.83(m,1H,HNCHCH2C6H5) , 3.64(s,3H,OCH3), 4.26-4.30 (m,2H,CH2C6H5), 7.18-7.23 (m,5H,C6H5 ) FTIR-
8400S; v(C=O) peak 1688 cm– 1,v( N-H) peak 3065 cm-1
2. BOC-PheTyr-OMe:1.33-1.39(m,9H,(CH3)3CO), 2.54-2.66(m,2H,CH2C6H5),2.76-2.92(m,2H,CH2C6H4OH), 3.63 (s,3H,OCH3 ), 4.26-4.28 (m,1H,NHCHCH2C6H5 ), 4.57-4.59 (m,1H,NHCHCH2C6H4OH ), 6.68-6.70 (m, 5H,C6H5), 7.16-7.29 (m,4H,C6H4OH ) FTIR-8400S; v(C=O) peak 1688cm-1,v( N-H) peak 3063 cm-1
3. BOC-PhePro-OMe: 1.27-1.47 (m,9H,(CH3)3CO ), 1.89-2.18 (m,2H,CH2C6H5 ), 2.93-3.46 (m,4H,NCH(CH2)2 ),3.57-3.63 (m, 1H,NCH(CH2)3), 3.70(s, 3H,OCH3), 4.67-5.03 (m,1H,NHCOCHCH2C6H5), 7.20-7.28 (m,5H,C6H5 ) FTIR-8400S; v(C=O) peak 1642 cm-1, v( N-H) peak 3431 cm-1
4. PheLeu OMe: v(C=O) peak 1672cm-1, v( N-H) peak 3289 cm-1
5. PheTyr-OMe: v(C=O) peak 1671cm-1, v( N-H) peak 3178 cm-1
6. PhePro-OMe: v(C=O) peak 1699cm-1, v( N-H) peak 3144 cm-1
Antimicrobial Activity
Table 1. Antimicrobial activity of protected dipeptides:
| S. No. | Compound name | Concentration | Aspergillusfumigatus(NCIM902) | Pencilliumchrysogenum(NCIM738) | E.coli(NCIM2563) | Salmonellatyphimurium(NCIM2501) | CH3OH | Ciprofloxacin(standard) |
| 1 | BOC-PheLeu-OMe | 250ppm | 2mm | X | 1mm | X | X | 20mm |
| 500ppm | 3mm | X | 2mm | X | X | 24mm | ||
| 1000ppm | 5mm | 3mm | 3mm | 1mm | X | 24mm | ||
| 2 | BOC-PheTyr-OMe | 250ppm | 1mm | 2mm | X | X | X | 18mm |
| 500ppm | 1mm | 3mm | X | X | X | 18mm | ||
| 1000ppm | 4mm | 8mm | 4mm | 2mm | X | 20mm | ||
| 3 | BOC-PhePro-OMe | 250ppm | 3mm | X | 3mm | 3mm | X | 22mm |
| 500ppm | 7mm | X | 3mm | 6mm | X | 20mm | ||
| 1000ppm | 11mm | 2mm | 8mm | 10mm | X | 20mm | ||
Table 2. Antimicrobial activity of deprotected dipeptides:
| S. No. | Compound name | Concentration | Aspergillusfumigatus(NCIM902) | Pencilliumchrysogenum(NCIM738) | E.coli(NCIM2563) | Salmonellatyphimurium(NCIM2501) | CH3OH | Ciprofloxacin(standard) |
| 1 | PheLeu-OMe | 250ppm | 1mm | 3mm | 2mm | X | X | 22mm |
| 500ppm | 2mm | 5mm | 4mm | X | X | 26mm | ||
| 1000ppm | 4mm | 8mm | 7mm | X | X | 24mm | ||
| 2 | PheTyr-OMe | 250ppm | 3mm | 2mm | X | X | X | 18mm |
| 500ppm | 4mm | 4mm | 2mm | X | X | 19mm | ||
| 1000ppm | 5mm | 6mm | 3mm | X | X | 20mm | ||
| 3 | PhePro-OMe | 250ppm | 3mm | 2mm | 4mm | X | X | 22mm |
| 500ppm | 4mm | 1mm | 5mm | X | X | 25mm | ||
| 1000ppm | 4mm | 5mm | 6mm | X | X | 27mm | ||
 |
Figure1: The disk represents the antimicrobial activity of PheLeu-OMe with their zone of inhibition at three concentrations of 250, 500 and 1000 ppm in comparison to standard cipro floxac in (± 30 mm) and solvent (Methanol) control Click here to View figure |
The antimicrobial activity of protected and deprotected dipeptides against fungal and bacterial strains is shown in table 1 and 2. Among protected dipeptides BOC-PhePro-OMe shows maximum potency against fungi Aspergillus fumigatus with inhibition zone diameter of 11mm at 1000 ppm (standard 20mm).This compound is not so effective against Pencillium chrysogenum. But it shows good antibacterial activity against gram- negative strains of E.coli(8mm) and Salmonella typhimurium (10 mm). This compound shows moderate potency against Aspergillus fumigatus, E.coli and Salmonella typhimurium at 250 and 500 ppm concentrations.
BOC-PheLeu-OMe is negligibly effective against Aspergillus fumigatus at all concentrations (250, 500 and 1000 ppm) with zonal diameter of 1mm, 2mm and 4mm respectively while standard has diameter of 20mm-24 mm.
Protected dipeptide BOC-PheTyr-OMe show moderate activity against fungal strain Pencillium chrysogenum at 1000 ppm (8mm).It is not effective against Aspergillus fumigatus, E.coli and Salmonella typhimuriumat concentrations of 250, 500 and 1000 ppm.
Deprotected dipeptides also show antibacterial and antifungal activities against the four strains. All the three deprotected dipeptides PheLeu-OMe, PheTyr-OMe, PhePro-OMe show moderate antifungal activity against Aspergillus fumigatusat concentrations of 250 ppm, 500 ppm and 1000 ppm. They are not showing any activity against Salmonella typhimurium even at higher concentration of 1000 ppm.Deprotected dipeptidesPheLeu-OMe, PheTyr-OMe, PhePro-OMe are showing good antifungal activity against fungal strain Pencillium chrysogenumat higher concentration (1000 ppm) with zonal diameter of 8mm, 6mm and 5mm (standard 22-27mm). They are also effective against gram-negative bacteria E.coliat 1000 ppm with inhibition zone diameter of 7mm, 3mm and 6mm respectively.
Conclusion
With the increase in pollution all over the world, various infectious diseases are emerging;also the microorganisms are be coming resistant to existing antibiotics. Therefore it is becoming a challenge for researchers to develop new methods for the treatment of these diseases and microorganisms11.From the results, we found that these protected and deprotected dipeptides exhibit antimicrobial activities at different concentrations. Out of these compounds BOC-PhePro-OMe and PheLeu-OMe(fig 1) are showing good antimicrobial activity against biologically important pathogens so these can be used as potent therapeutic agents after their further investigations.
Acknowledgment
We are grateful to Dr. Joginder Singh for antimicrobial activity, Lovely Professional University for lab facility and SAIF, Panjab University Chandigarh for NMR facility.
References
- Bryan, A.; Joseph, L.;Bennett,J.A.;Jacobson, H.I.; Andersen, T. T. Peptides 2011, 32,2504-2510
- Gentilucci, L.; Cardillo, G.; Tolomelli, A.; Squassabia, F.; Chiriano, D.M.G. Comb Chem High Throughput Screen 2009,12, 929-939
- Young,P.E.; Campbell, A. Journal of chemical education 1982, 59, 701-702
- Anderson, G.W.; Zimmerman, J.E.; Callahan, F.M.J. Am. Chem. Soc. 1967,89, 5012-5017
- Joullie, M.M.; Lassen, K.M. Arkivoc2010, 8, 189-250
- Hameurlaine, A.; Saved, W.A.; Rahman, A.H.A. Monatsh Chem.2008, 139, 1507-1511
- Zinelaabidine, C.; Souad, O.; Berredjem, J.Z.; Eddine, A.N.International journal of Chemistry 2012,4, 73-79
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M.Am.J.Clin.Pathol. 1996,45, 493-496
- Hossain, S.M.; Easmin, S.; Islam, S.M.; Mamunur, R.J.Pharm.Pharmacol. 2004,56, 1519-1520
- Nagarajan,K.; Senthamarai, R.; Devi, K.; Deepashalini, S.; Anandh, N.; Krishnaveni,P.; Mazumder, A.; Ghosh, L.K.; Umadevi, G.Journal of cell and tissue research 2008,8,1265-1269
- Strohl W. R., Biotechnology of Antibiotics,Vol. 82.;Marcel Dekker. New York, (1997)

This work is licensed under a Creative Commons Attribution 4.0 International License.









