Synthesis and Characterisation of Phenanthroline Adducts of Pb(II) Complexes of BisN-alkyl-N-ethyldithiocarbamates
Normah Awang1, Nadia Atiqah Nordin2, Nurlina Rashid2 and Nurul Farahana Kamaludin2
1Environmental Health and Industrial Safety Programme, School of Diagnostic and Applied Health Sciences, Faculty of Health Sciences, Universiti Kebangsaan Malaysia, Jalan Raja Muda Abdul Aziz, 50300 Kuala Lumpur,
2School of Chemical Science and Food Technology, Faculty of Science Technology, Universiti Kebangsaan Malaysia, 43600 Bangi, Selangor, Malaysia
DOI : http://dx.doi.org/10.13005/ojc/310138
Article Received on :
Article Accepted on :
Article Published : 04 Mar 2015
Dithiocarbamates are a class of metal-chelating compounds with various applications in medicine. They have been used for the treatment of bacterial and fungal infections, possible treatment of AIDS, and most recently cancer. Dithiocarbamates are well-known compounds to bind strongly and selectively to many metal ions.Dithiocarbamateligands readily form chelates withmetal ions through its two donor sulphur atoms.A new series of plum bum(II) complexes with mixed ligands, dithiocarbamate, and 1,10-phenanthroline were successfully synthesised using in situ method. Microelemental analysis data of the complexes were in agreement with the general formula, Pb[S2CNR’R”]3phen (R’ = ethyl, R” = butyl, cyclohexyl; phen = 1,10-phenanthroline). Infrared spectra of the complexes showed that the thioureide ν(C-N) band was in the region 1450–1500 cm-1. The unsplitting band of ν(C-S) in the region 930–1000 cm-1indicatedthe bidentate nature of the chelated dithiocarbama to ligands. The 13C NMR chemical shift of the carbon atom of the N-CS2 group appeared in the range of 202–207 ppm.
KEYWORDS:dithiocarbamate; thioureide; plumbum; chemical shift; phenanthroline
Download this article as:| Copy the following to cite this article: Awang N, Nordin N. A, Rashid N, Kamaludin N. F. Synthesis and Characterisation of Phenanthroline Adducts of Pb(II) Complexes of BisN-alkyl-N-ethyldithiocarbamates. Orient J Chem 2015;31(1). |
| Copy the following to cite this URL: Awang N, Nordin N. A, Rashid N, Kamaludin N. F. Synthesis and Characterisation of Phenanthroline Adducts of Pb(II) Complexes of BisN-alkyl-N-ethyldithiocarbamates. Orient J Chem 2015;31(1). Available from: http://www.orientjchem.org/?p=7550 |
Introduction
Dithiocarbamates are highly versatile monoanionic chelating ligands that form stable complexes with all the transition elements and the
majority of main group elements, lanthanide and actinideelements(Hogarth 2012). Dithiocarbamates are prepared from primary or secondary amines.Depending upon the nature of the cation, dithiocarbamates can show good solubility in water or organic solvents. Dithiocarbamates are lipophilic and generally bind to metals in a symmetrical chelate fashion.There are also other coordination modes, but monodentate and anisobidentate modes are the two most prevalent ones.
Dithiocarbamates stabilise metals in a wide range of oxidation states, this being attributed to the existence of soft dithiocarbamate and hard thioureide resonance forms, the latter formally resulting from delocalisation of the nitrogen lone pair onto the sulphurs, and consequently their complexes tend to have a rich electrochemistry (Onwudiwe 2014). Tetraethyl thiuramdisulphide (disulfiram or antabuse) has been used as a drug since the 1950 s, but it is only recently that dithiocarbamate complexes have been explored within the medicinal domain. Over the past two decades, anticancer activity of gold and copper complexes has been noted.Technetium and copper complexes have also been used in PET imaging. Copper complexes have also been investigated as SOD inhibitors. Other than that, dithiocarbamates have been used to treat acute cadmium poisoning.
Tetrahedral metal dithiocarbamate complexes are known to expand their coordination numbers by adding neutral nitogen ligands. The
crystal structure on mixed ligand complexes involving cadmium dithiocarbamate with nitrogen bidentate ligand, 1,10-phenantrhroline has been reported (Lai et al. 2003; Ali et al. 2009).All the adducts contain discrete molecular units with CdS 4N 2 chromophore in a distorted octahedral geometry.
In our previous work, we have reported several compounds and adduct of dithiocarbamate complexes (Awang et al. 2011; Awang et al. 2010). As an extension of this research, here we report the synthesis of two new Pb(II) dithiocarbamate compounds, which arePb[S 2 CN(C 2 H 5 )(C 4 H 9 )] 2 (compound 1) and Pb[S 2 CN(C 2 H 5 )(C 6 H 11 )] 2 (compound 2). We also report their adducts, which are Cd[S2CN((C2H5)(C4H9)]]2(phen) (compound 3) and Cd[S2CN(C2H5)(C6H11)]2(phen) (compound 4), together with their elemental analysis and spectroscopic characterisation.
Materials and Method
Reagents
Secondary amines (N-butyl-N-ethylamine and N-cyclohexyl-N-ethylamine) and ethanol (95%) were obtained from Fluka Chemical. Carbon disulphide and methanol (99.5%) were from Ajax Chemical Ltd., and dichloromethane(99.5%) from R & M Chemical (99.5%). PbCl 2 chloride was purchased from Merck. All the chemicals were used as supplied without purification.
Synthesis of dithiocarbamate compounds Synthesis of Plumbum(II) N-alkyl-N- ethyldithiocarbamate Compounds (Compound 1 and 2)
Compound 1 and 2 were synthesised by the method given by Thirumaran et al. (1998) with some modifications.Carbon disulphide (30 mmol) in ethanol was slowly added to an ethanolic solution of N-alkyl-N-ethylamine (30 mmol). The reaction temperature was controlled under 4°C. The mixture was stirred about 1h,and a pale yellow solution formed.After that, 30 mmol of plumbum(II) chloride in ethanol solution was added, and the yellowish precipitate was obtained. The precipitate was filtered and washed with cold ethanol. The reaction scheme for the above preparation is given in Figure 1.
Synthesis of Plumbum(II) N-alkyl-N- ethyldithiocarbamate (Phen) Compounds
(Compound 3 and 4)
Compound 3 and 4 were prepared by adding equimolar of compound 1 or compound 2 and bidentate nitrogen ligand (1,1-phenanthroline)
in the mixture of ethanol and chloroform solutions. The resulting mixture was stirred for 1 h and allowed to evaporate at room temperature. The pale yellow precipitate formed and was washed with cold ethanol and dried in vacuo. The reaction scheme to synthesise compounds 3 and 4 is shown in Figure 2.
Physical Measurements
Elemental analysis (CHNS) was carried out on a CHNS/O Fison Model EA 1108 elemental analyser. Melting points were determined using an Electrothermal 9100 instrument.The infrared spectra were recorded on a Perkin Elmer FT-IR Model Spectrum GX spectrophotometer; using KBr discs for the wave number range between 4000 and 400 cm-1 and nujol in polyethylene tablets for the wave number range between 400 and 250 cm-1.
The 13C NMR spectra werere corded using a Jeol JNM-LA 400 spectrometer with TMS as the internal standard.
Results and Discussion
Physical and Elemental Analysis
The synthesised compounds were solid and stable in air. The elemental analysis dan melting point data for the title compounds are given in Table 1.Elemental analysis was done to compare the observed values with predicted values of percentage of the carbon, hydrogen, nitrogen, and sulphur. The elemental analysis data were in good agreement with the expected values. These data showed that the chemical formula for the compound 1 and 2 was Pb[S2CN(CH2CH3)(R)]2 (R = C6H11 or C4H9 ), whereas for compound 3 and 4 was Pb[S2CN(CH2CH3 )(R)]2.C12H8 N2 (R = C6 H11 or C4H9). The proposed structures for compound 1 and 2 are shown in Figure 2.All the compounds were Nonygroscopic and stable at room temperature.
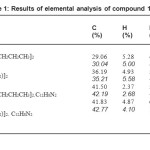 |
Table1: Results of elemental analysis of compound 1 to 4 Click here to View table |
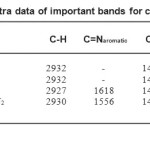 |
Table2: Infrared spectra data of important bands for compound 1 to 4 Click here to View table |
These compounds were insoluble or sparingly soluble in most common organic solvent and quite soluble in chloroform.They had sharp melting point. The small range of their melting point showed that these compounds had a good purity (Awang et al. 2010).
Infrared Spectroscopy
The characteristic infrared absorption frequencies (cm-1) and assignment of important absorption bands of the compound 1 to 4 are listed
in Table 2. The significant absorptions in the dithiocarbamate complexes mainly are due to the v(C- N) and v(C-S)stretching modes (Geeta and Thirumaran 2008). The v(C- N)was used as a measure of the contribution of the thioureide group to the structure of the dithiocarbamate. Thev(C- N) appeared at an intermediate value between the two extremes of 1250–1350 cm-1) and (1650–1690 cm-
1) (Baggio et al. 1992). IR spectra of the complexes showed that the shift of the thioureide bands to lower frequencies compared to the parent bisdithiocarbamate was due to the increase in coordination number around the metal ion (Awang
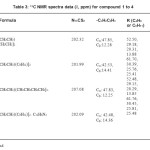 |
Table3: 13C NMR spectra data (δ, ppm) for compound 1 to 4 Click here to View table |
et al. 2011, Manohar et al. 2012). The thioureide band was well differentiated from the ring frequencies associated with N-donors, which were observed in the 1600–1500 cm-1. The v(C-S) bands appear around 990 cm-1 in all the complexes without any splitting, supporting the bidentate coordination of the dithiocarbamate moiety (Manohar et al. 1998). Figure 4(a-b) shows the IR spectra of compound 1 and 3.
The 13C NMR spectra data of compound 1 to 4 are shown in Table 4. The assignments of –CSS group in the investigated compounds were straight ward as observed in the range of 201.99–207.08 ppm, indicating the coordination of sulphur to the lead atom.The N13CS2 chemical shift was the most important signal in the 13 C NMR spectra for the dithiocarbamate compounds. The 13C NMR spectra of compound 3 and 4 were observed at 207.08 and 202.09 ppm, respectively, which shifted to down field compared to the parent compound (compound 1 and 2). The shift could be explained by an increase of π-bond order in the whole NCS2 moiety. This means that the chelation of the bidentate nitrogen ligands to the plumbum atom promoted the delocalisation of the unshared electron pair in the
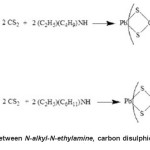 |
Figure1: Reaction scheme between N-alkyl-N-ethylamine, carbon disulphide, and plumbum(II)nitrate Click here to View figure |
nitrogen atom in the dithiocarbamate group (Awang et al. 2011).The occurrence of six resonances in the range of 123–151 and 113–154 ppm for compound 3 and compound 4, respectively,was defined as benzene carbon signals of 1,10- phenanthroline ligand. Meanwhile, the signals of ethyl group (Cαand Cβ) were seen in the range of 12.25–47.85 ppm.
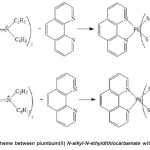 |
Figure2: Reaction scheme between plumbum(II) N-alkyl-N-ethyldithiocarbamate with1,10-phenanthroline Click here to View figure |
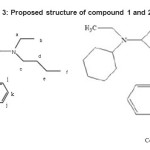 |
Figure3: Proposed structure of compound 1 and 2 Click here to View figure |
 |
Figure4: Proposed structure of compound 3 and 4 Click here to View figure |
 |
Figure5: IR spectra of compound 1 (a) and compound 3 (b) Click here to View figure |
Conclusion
The parent plumbum(II) dithiocarbamate and their nitrogenous adduct have been successfully synthesised and characterised. The IR spectra data of the comppunds showed that the thioureide band values were lower than the values observed for the parent dithiocarbamate compounds. A reduction in the thioureide strectcing frequency was due to the increase in coordination around the cadmium ion and the resultant increase in electron density.
Acknowledgement
We would like to thank the Malaysia Ministry of Higher Education for the financial support (Grant Code:06-01-02-SF0887). We would also like to thank for technical support from the laboratory assistants of Faculty Science and Technology, Universiti Kebangsaan Malaysia.
References
- Manohar, A., Ramalingam, K. Karpagavel, K. &Kulandisamy, A. International Journal of ChemTech Research. 2012. 4(3): 1023-1032.
- Hogarth, G. Metal-dithiocarbamate complexes: chemistry and biological activity. Mini Rev. Med. Chem. 2012, 12(12): 1202-1215.
- Lai, C.S. and Tiekink, E.R.T.Bis(N, N-ditehtyldithiocarbamato)(2,9-dimethyl-1,10-phenanthroline) cadmium(II). Appl. Organometalic Chem. 2003, 17: 139-140.
- Ali, B.F., Al-Far, R., Zaghal, M.H., Judeh, Z. and Haddad, S.F. Cadmium(II) diallyldithiocarbamato complexes with 2,2’bipyridine and 1,10-phenanthroline: Spectroscopic and crystal structure analysis. J. Coord. Chem. 2009, 62: 2028-2036.
- NormahAwang, Ibrahim Baba, Bohari M. Yamin and Azhar Abdul Halim. Preparation, Characterization and Antimicrobial Assay of 1,10-Phenanthroline and 2,2’-Bipyridyl adducts of Cadmium(II) N-Sec-Butyl-NPropyldithiocarbamate: Crystal Structure of Cd[S2CN(i-C4H9)(C3H7)]2(2,2’-Bipyridyl).World Applied Sciences Journal 2011, 12(9): 1568-1574.
- NormahAwang, Ibrahim Baba, Bohari M. Yamin and SeikWeng Ng.Bis(N-isobutyl-N-propyl-dithiocarbamato-κ2 S,S’)zinc(II). ActaCrystallographica Section E: Structure Reports Online. 2010, 66(2):m215.
- Manohar, A., Venkatachalam, V., Ramalingam, K. Thirumaran, DS. Bocelli, G. &Cantoni, A.Synthesis, spectral, and single crystal X-ray structural studies on (2,2′-bipyridyl)bis(dimethyldithiocarbamato) zinc(II) and (1,10-phenanthroline) bis(dimethyldithiocarbamato)zinc(II), Journal of Chemical Crstallography. 1998, 28(12): 861-866.
- Onwudiwe, D.C., Arfin, T. and Strydom, C.A. Fe(II) and Fe(III) Complexes of N-Ethyl-N-Phenyldithiocarbamate: Electrical Conductivity Studies and Thermal Properties. ElectrochimicaActa, 2014, 127:283-289.
- Geetha, N. &Thirumaran, S. Characterization studies and cyclic voltammetry on nickel(II) amino acid dithiocarbamates with triphemylphosphine in the coordination sphere. J. Ser. Che. Soc. 2008, 73(2): 169-177.
- Thirumaran, S., V. Venkatachalam, A. Manohar, K.Ramalingam and G. Bocelli.. Synthesisand characterization of bis(N-Methyl-N-ethanol-dithiocarbamato)M(II) (M = Zn, Cd, Hg) andBis(N,N-(iminodiethylene)- bisphthalimidedithiocarbamato)M(II) (M = Zn, Cd,Hg) complexes. Single crystal X-ray structure of bis(di(2-hydroxyethyl)-dithiocarbamato)zinc(II). J.Coord. Chem., 1998, 44: 281-288.

This work is licensed under a Creative Commons Attribution 4.0 International License.









