Solvent-Free Synthesis of 1,2,4,5-Tetrasubstituted Imidazoles Using Nano Fe3O4@SiO2-OSO3H as a Stable and Magnetically Recyclable Heterogeneous Catalyst
Sepideh Yadegarian, Abolghasem Davoodnia* and Ahmad Nakhaei
Department of Chemistry, Mashhad Branch, Islamic Azad University, Mashhad, Iran
DOI : http://dx.doi.org/10.13005/ojc/310173
Article Received on :
Article Accepted on :
Article Published : 30 Mar 2015
Nano-Fe3O4 encapsulated-silica particles bearing sulfonic acid groups (Fe3O4@SiO2-OSO3H) has been used as an efficient and magnetically separable heterogeneous catalyst for the synthesis of 1,2,4,5-tetrasubstituted imidazoles by one-pot, four-component reaction of benzil, aromatic aldehydes, primary amines, and ammonium acetate under neat conditions. The effect of various reaction parameters, such as reaction temperature, catalyst loading and solvent effect, were studied. The desired products are obtained in relatively short reaction times with high yields. Importantly, the catalyst could be easily separated from the reaction mixture by a permanent magnet and reused for several times without the significant loss of its activity.
KEYWORDS:Fe3O4@SiO2-OSO3H; Magnetically separable; Solvent-free conditions; 1;2;4;5-Tetrasubstituted imidazoles
Download this article as:| Copy the following to cite this article: Yadegarian S, Davoodnia A, Nakhaei A. Solvent-Free Synthesis of 1,2,4,5-Tetrasubstituted Imidazoles Using Nano Fe3O4@SiO2-OSO3H as a Stable and Magnetically Recyclable Heterogeneous Catalyst . Orient J Chem 2015;31(1). |
| Copy the following to cite this URL: Yadegarian S, Davoodnia A, Nakhaei A. Solvent-Free Synthesis of 1,2,4,5-Tetrasubstituted Imidazoles Using Nano Fe3O4@SiO2-OSO3H as a Stable and Magnetically Recyclable Heterogeneous Catalyst . Orient J Chem 2015;31(1). Available from: http://www.orientjchem.org/?p=8145 |
Introduction
In synthetic organic chemistry, the one-pot multicomponent reaction (MCR) strategy has become a powerful tool to improve the efficiency of a reaction wherein a reactant is subjected to successive chemical reactions in one vessel. This process consists of two or more synthesis steps which are carried out without isolation of any intermediate. The overall advantages of this strategy involve: i) use of commercially available starting materials; ii) avoiding a lengthy separation of the intermediates; iii) saving time and resources; iv) achieving increase in the chemical yield1-3. The development of new MCRs and improvement of known MCRs are therefore areas of considerable current interest. One such reaction is the synthesis of 1,2,4,5-tetrasubstituted imidazoles. The imidazole ring system is one of the most important substructure found in a large number of natural products and pharmacologically active compounds4,5. Different substituted imidazoles show variable biological activities such as antibacterial, anti-allergic, anti-inflammatory, antitumor analgesic, glucagon receptor antagonism therapeutic agents, and pesticides6-9. Also, some of them are known as inhibitors of P38 MAP10 kinase and B‐Raf kinase11. Recent advances in green chemistry and organometallic catalysis has extended the application of imidazoles as ionic liquids12-14 and N-heterocyclic carbenes15.
Numerous methods for the synthesis of 1,2,4,5-tetrasubstituted imidazoles have been reported. They can be accessed by cyclization of α-(N-acyl-N-alkylamino-β-ketoamides in refluxing AcOH16, hetero-Cope rearrangement17, condensation of a 1,2-diketone with an aryl nitrile and primary amine under microwave irradiation18, and N-alkylation of trisubstituted imidazoles19. Another direct method involves a four-component condensation of 1,2-diketones or α‐ hydroxyketones with aldehydes, primary amines, and ammonium acetate in the presence of a catalyst such as TFA in ionic liquid [Bpy]BF420, montmorillonite K10 supported titanium21, L-proline22, InCl3.3H2O23, BF3-SiO224, Brønsted acidic ionic liquid25, carbon-based solid acid26, K5CoW12O40.3H2O27,p-dodecylbenzenesulfonic acid28, FeCl3/montmorillonite K10 under microwave irradiation29, NH4H2PO4/Al2O330, NaHSO4/SiO231, and H6P2W18O62.24H2O/SiO232. Each of these methods has its own merit, however, all of the reported methods (except for the reaction catalyzed by FeCl3/montmorillonite K10 under microwave irradiation) require relatively prolonged reaction time. Also, some of these catalysts are expensive or having other problems like requirement of solvents for synthesis, and low yields of the products. Furthermore, the synthesis using homogeneous catalysts have major problem of catalyst recovery and reuse. To avoid these limitations, the discovery of a new and efficient catalyst with high catalytic activity, short reaction time, recyclability, and simple reaction work-up for the synthesis of 1,2,4,5-tetrasubstituted imidazoles is of prime interest.
In recent years economic and environmental concerns encourage the application of heterogeneous catalysts to carry out various organic transformations33-36. These catalysts make the processes clean, safe, high-yielding and inexpensive. In recent years, the use of heterogeneous nanocatalysts have been receiving growing attention, because they have high catalytic activities due to their large specific surface area37,38. Recently, nano-Fe3O4 encapsulated-silica particles bearing sulfonic acid groups (Fe3O4@SiO2-OSO3H) have been prepared and used as an efficient acid catalyst in some organic reactions39-42. They can be readily separated from the reaction mixture by a permanent magnet and reused several times. The process is more effective than filtration and centrifugation in preventing loss of the solid catalyst. However, there are no reports on the use of Fe3O4@SiO2-OSO3H as an acidic heterogeneous catalyst for the synthesis of 1,2,4,5-tetrasubstituted imidazoles.
As a consequence of our interest in developing new efficient synthetic methodologies 43-47 for the synthesis of bioactive molecules and owing to the biological significance of 1,2,4,5-tetrasubstituted imidazoles, we wish to report herein a solvent-free synthesis of 1,2,4,5-tetrasubstituted imidazoles catalyzed by Fe3O4@SiO2-OSO3H, as a green and effective magnetically recyclable heterogeneous catalyst (Scheme 1).
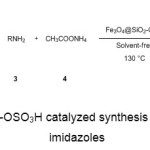 |
Scheme1: Fe3O4@SiO2-OSO3H catalyzed synthesis of 1,2,4,5-tetrasubstituted imidazoles Click here to View Scheme |
Experimental
All chemicals were available commercially and used without additional purification. The melting points were recorded using a Stuart SMP3 melting point apparatus. The IR spectra of the productswere obtained with KBr disks, using a Tensor 27 Bruker spectrophotometer. The 1H NMR (400 & 500 MHz) spectra were recorded using Bruker 400 & 500 spectrometers.
General Procedure for the Synthesis of 1,2,4,5-Tetrasubstituted Imidazoles 5a-m Using Fe3O4@SiO2-OSO3H as Catalyst
A mixture of benzil 1 (1 mmol), an aromatic aldehyde 2 (1 mmol), a primary amine 3 (1 mmol), ammonium acetate 4 (1 mmol), and Fe3O4@SiO2-OSO3H (0.10 g) was heated in the oil bath at 130 °C for 15-60 min. The reaction was monitored by thin-layer chromatography (TLC). Upon completion, hot ethanol was added to the reaction mixture and the catalyst was separated using an external magnet (Fig 1.). The catalyst was washed with a small portion of hot ethanol (10 mL). The combined filtrate was concentrated by half and allowed to stand at r.t.. The precipitated solid was collected by filtration, and recrystallized from ethanol 96% to give compounds 5a-m in high yields.
 |
Figure1: The reaction mixture in the synthesis of 5a after adding hot ethanol in the presence of a magnetic field Click here to View figure |
Results and Discussion
For our investigations, the catalyst Fe3O4@SiO2-OSO3H was prepared according to the literature procedure39,40. The FT-IR spectrum of the catalyst (Fig. 2a) exhibits the Fe-O vibration 39,40 at 587 cm-1. The bands at 1091 cm-1, 797 cm-1 and 463 cm-1 were attributed to the Si-O-Si asymmetric and symmetric stretching vibrations as well as bending vibration, respectively40. The spectrum also shows the SO2 symmetric and asymmetric stretching modes in 1100-1250 cm-1 that were covered by a stronger absorption of Si-O bond 40,43 at 1091 cm-1. Also, the absorption band at 1637 cm-1 and the broad band at around 3000-3700 cm-1 were attributed to stretching vibration of the adsorbed water40,41. The density of the SO3H groups was measured using KOH (0.01 mol L-1) as titrant by acid-base potentiometric titration. The amount of SO3H in the catalyst was 1.5 mmol/g.
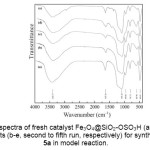 |
Figure2: FT-IR spectra of fresh catalyst Fe3O4@SiO2-OSO3H (a, first run), and recovered catalysts (b-e, second to fifth run, respectively) for synthesis of compound 5a in model reaction. Click here to View figure |
In the effort to develop an efficient and environmentally benign method for synthesis of 1,2,4,5-tetrasubstituted imidazoles we initiated our studies by adding a catalytic amount of Fe3O4@SiO2-OSO3H to a mixture of benzil (1 mmol), benzaldehyde (1 mmol), aniline (1 mmol), and ammonium acetate (1 mmol), as model reaction, in different solvents and under solvent-free conditions (Table 1). We were pleased to see that the reaction was efficiently catalyzed by Fe3O4@SiO2-OSO3H under solvent-free conditions at elevated temperature leading to a high yield of product 5a. The reaction conditions were then optimized by conducting the reaction at different temperatures and using different amounts of catalyst. The results are summarized in Table 1. Low yield of the product was obtained in the absence of the catalyst at 130 °C after 120 min (entry 1) indicating that the catalyst is necessary for the reaction. The best result was obtained when the reaction was run at 130 °C in the presence of 0.10 g Fe3O4@SiO2-OSO3H under solvent-free conditions (entry 13).
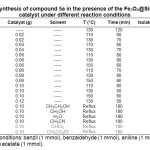 |
Table1: Synthesis of compound 5a in the presence of the Fe3O4@SiO2-OSO3H catalyst under different reaction conditions Click here to View table |
Using these optimized reaction conditions, and in order to evaluate the generality of this model reaction, we extended the reaction of benzil and ammonium acetate with a range of other aromatic aldehydes and primary amines (Table 2). As shown, in all cases, the expected products were obtained in high yields over relatively short reaction times. Easy separation of obtained products from the catalyst makes this method useful for the synthesis of 1,2,4,5-tetrasubstituted imidazoles.
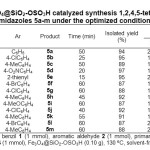 |
Table2: Fe3O4@SiO2-OSO3H catalyzed synthesis 1,2,4,5-tetrasubstituted imidazoles 5a-m under the optimized conditions Click here to View table |
In order to know the catalytic efficiency of Fe3O4@SiO2-OSO3H, the data reported in the literature for the synthesis of 1,2,4,5-tetrasubstituted imidazoles using different type of the catalysts was compared with Fe3O4@SiO2-OSO3H. As shown in Table 3, our reaction conditions gave a shorter reaction time than all the other conditions (except for the reaction catalyzed by FeCl3/montmorillonite K10 under microwave irradiation) and gave high yields of the desired products with very simple work-up procedure.
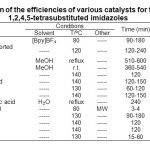 |
Table3: Comparison of the efficiencies of various catalysts for the synthesis of 1,2,4,5-tetrasubstituted imidazoles Click here to View table |
In view of environmental friendly methodologies, recovery and reuse of the catalyst is highly preferable. Upon completion of the reaction, hot ethanol was added and the catalyst was recovered from the reaction mixture simply by applying an external permanent magnet. The recovered catalyst was washed with hot ethanol and then dried under vacuum at 50 °C for 1 h before being reused in the same reaction. The catalyst could be used at least five times with only slight reduction in the catalytic activity. The yields obtained were from 94, 93, 93, 91 and 90% in the first, second, third, fourth and fifth run respectively. Furthermore, retention of the structure of the catalyst was confirmed by comparing the FT-IR spectra of the recovered catalysts (Fig. 2b-e) with that of the fresh catalyst (Fig. 2a), for model reaction. As shown, these spectra are almost identical.
Conclusion
In summary, in this paper a highly efficient, neat and green method with a simple work-up procedure for the synthesis of 1,2,4,5-tetrasubstituted imidazoles via one-pot, four-component reaction of benzil, aromatic aldehydes, primary amines, and ammonium acetate has been reported. The catalyst could be readily recovered using a simple external magnet and reused several times without any significant loss in activity. Other attractive features of this protocol are high yields, relatively short reaction times, avoiding the use of harmful organic solvents in the reaction and reusability of the catalyst. The catalyst can be used at least five times without substantial reduction in its catalytic activity.
Acknowledgement
The authors express their gratitude to the Islamic Azad University, Mashhad Branch for its financial support.
References
- Bagley, M. C.; Lubinu M. C. Top. Heterocycl. Chem. 2006, 31.
- Chebanov, V. A.; Desenko, S. M. Chem. Heterocycl. Compd. 2012, 48, 566.
- Zeinali-Dastmalbaf, M.; Davoodnia, A.; Heravi, M. M.; Tavakoli-Hoseini, N.; Khojastehnezhad, A.; Zamani, H. A. Bull. Korean Chem. Soc. 2011, 32, 656.
- Heers, J.; Backx, L. J. J.; Mostmans, J. H.; Van Cutsem, J. J. Med. Chem. 1979, 22, 1003.
- Hunkeler, W.; Mohler, H.; Pieri, L.; Polc, P.; Bonetti, E. P.; Cumin, R.; Schaffner, R.; Haefely, W. Nature 1981, 290, 514.
- Ghosh, R.; De, B. Int. J. Pharm. Sci. Rev. Res. 2013, 23, 237.
- Rani, N.; Sharma, A.; Singh, R. Mini-Rev. Med. Chem. 2013, 13, 1812.
- Ahsan, I.; Sharma, K. K.; Sharma, A.; Khan, S. A.; Khan, U. Pharm. Chema. 2014, 6, 320.
- Chang, L. L.; Sidler, K. L.; Cascieri, M. A.; de Laszlo, S.; Koch, G.; Li, B.; Mac‐Coss, M.; Mantlo, N.; O’Keefe, S.; Pang, M.; Rolando, A.; Hagmann, W. K. Bioorg. Med. Chem. Lett. 2001, 11, 2549.
- Murry, J. A. Curr. Opin. Drug. Discov. Dev. 2003, 6, 945.
- Takle, A. K.; Brown, M. J. B.; Davies, S.; Dean, D. K.; Francis, G.; Gaiba, A.; Hird, A. W.; King, F. D.; Lovell, P. J.; Naylor, A.; Reith, A. D.; Steadman, J. G.; Wilson, D. M. Bioorg. Med. Chem. Lett. 2006, 16, 378.
- Dupont, J.; de Souza, R. F.; Suarez, P. A. Z. Chem. Rev. 2002, 102, 3667.
- Davoodnia, A.; Khojastehnezhad, A.; Bakavoli, M.; Tavakoli‐Hoseini, N. Chin. J. Chem. 2011, 29, 978.
- Davoodnia, A.; Heravi, M. M.; Rezaei‐Daghigh, L.; Tavakoli‐Hoseini, N. Monatsh. Chem. 2009, 140, 1499.
- Bourissou, D.; Guerret, O.; Gabbai, F. P.; Bertrand, G. Chem. Rev. 2000, 100, 39.
- Zhang, C.; Moran, E. J.; Woiwode, T. F.; Short, K. M.; Mjalli A. M. M. Tetrahedron Lett. 1996, 37, 751.
- Lantos, I.; Zhang, W. Y.; Shui, X.; Eggleston, D. S. J. Org. Chem. 1993, 58, 7092.
- Balalaie, S.; Hashemi, M. M.; Akhbari, M. Tetrahedron Lett. 2003, 44, 1709.
- Davidson, D.; Weiss, M.; Jelling, M. J. Org. Chem. 1937, 2, 319.
- Wan, Y.; Liu, G. X.; Zhao, L. L.; Wang, H. Y.; Huang, S. Y.; Chen, L. F.; Wu, H. J. Heterocyclic Chem. 2014, 51, 713.
- Kannan, V.; Sreekumar, K. J. Mol. Catal. A: Chem. 2013, 376, 34.
- Samai, S.; Nandi, G. C.; Singh, P.; Singh, M. S. Tetrahedron 2009, 65, 10155.
- Sharma, S. D.; Hazarika, P.; Konwar, D. Tetrahedron Lett. 2008, 49, 2216.
- Sadeghi, B.; Mirjalili, B. B. F.; Hashemi, M. M. Tetrahedron Lett. 2008, 49, 2575.
- Davoodnia, A.; Heravi, M. M.; Safavi-Rad, Z.; Tavakoli-Hoseini, N. Synth. Commun. 2010, 40, 2588.
- Tavakoli-Hoseini, N.; Davoodnia, A. Chin. J. Chem. 2011, 29, 203.
- Nagarapu, L.; Apuri, S.; Kantevari, S. J. Mol. Catal. A: Chem. 2007, 266, 104.
- Das, B.; Kashanna, J.; Kumar, R. A.; Jangili, P. Monatsh. Chem. 2013, 144, 223.
- Raghuvanshi, D. S.; Singh, K. N. Indian J. Chem. 2010, 49B, 1394.
- Emrani, A.; Davoodnia, A.; Tavakoli-Hoseini, N. Bull. Korean Chem. Soc. 2011, 32, 2385.
- Karimi, A. R.; Alimohammadi, Z.; Azizian, J.; Mohammadi, A. A.; Mohammadizadeh, M. R. Catal. Commun. 2006, 7, 728.
- Karimi, A. R.; Alimohammadi, Z.; Amini, M. M. Mol. Divers. 2010, 14, 635.
- Agirrezabal-Telleria, I.; Gandarias, I.; Arias, P. L. Catal. Today 2014, 234, 42.
- Aoyama, T.; Takido, T.; Kodomari, M. Synlett, 2004, 13, 2307.
- Saburi, E.; Davoodnia, A.; Tavakoli-Hoseini, N. Synth. React. Inorg. Met-Org. Nano-Met. Chem. 2011, 41, 1063.
- Davoodnia, A.; Tavakoli-Nishaburi, A.; Tavakoli-Hoseini, N. Bull. Korean Chem. Soc. 2011, 32, 635.
- Rayati, S.; Abdolalian, P. Appl. Catal. A: Gen. 2013, 456, 240.
- Mirzaei, H.; Davoodnia, A. Chin. J. Catal. 2012, 33, 1502.
- Nemati, F.; Heravi, M. M.; Saeedirad, R. Chin. J. Catal. 2012, 33, 1825.
- Kiasat, A. R.; Davarpanah, J. J. Mol. Catal. A: Chem. 2013, 373, 46.
- Xiong, Y.; Zhang, Z.; Wang, X.; Liu, B.; Lin, J. Chem. Eng. J. 2014, 235, 349.
- Nemati, F.; Saeedirad, R. Chin. Chem. Lett. 2013, 24, 370.
- Ashrafi, M.; Davoodnia, A.; Tavakoli-Hoseini, N. Bull. Korean Chem. Soc. 2013, 34, 1508.
- Davoodnia, A. Bull. Korean Chem. Soc. 2011, 32, 4286.
- Davoodnia, A.; Zare-Bidaki, A.; Behmadi, H. Chin. J. Catal. 2012, 33, 1797.
- Davoodnia, A.; Khashi, M.; Tavakoli-Hoseini, N. Chin. J. Catal. 2013, 34, 1173.
- Davoodnia, A, Khashi, M, Tavakoli-Hoseini, N. Chin. J. Catal. 2014, 35, 1054. 4, 35, 1054.

This work is licensed under a Creative Commons Attribution 4.0 International License.









