Removal of Cd (II) and Hg(II) by chelating resin Chelex-100
Afaf Amara-Rekkab, Mohamed Amine Didi*
Laboratory of Separation and Purification Technologies, Department of Chemistry, Faculty of Sciences, BP 119, Tlemcen University — 13000, Algeria.
DOI : http://dx.doi.org/10.13005/ojc/310122
Article Received on :
Article Accepted on :
Article Published : 14 Mar 2015
A sensitive, simple method for the determination of amounts of mixture of Hg2+and Cd2+ by spectrophotometry was described based on the formation of the Hg2+- Cd2+- PAN complex in water media. Optimal conditions such as reagent amounts, and pH for the Hg2+- Cd2+ determination were reported. It was found that the 2:1 PAN- Hg2+- Cd2+ complex dominate at pH 13.0. In another hand, the sorption of mixture mercury (II) and cadmium (II) from aqueous medium on a chelating resin Chelex 100 was studied in batch mode. Since the extraction kinetic was obtained, with a mixture of 0.1 g of resin and 5 mL of mixture at 1 mmol/L of initial concentration, extraction equilibrium was reached within 20 min of mixing. The influence of some parameters such as initial mixture ions concentrations, initial pH of aqueous solution, ion strength and the amounts of resin have been studied at fixed temperature (20±1°C). The optimum pH value level for quantitative sorption was up to 2.6. The best performance obtained was 97.1% of extraction yield equivalent to 15.65 mg/g of resin. The pseudo-first- order, pseudo-second-order models and the intra-particle diffusion model were used to describe the kinetic data and rate constants were evaluated.
KEYWORDS:Mercury; Cadmium; spectrophotometry; PAN; Chelex 100
Download this article as:| Copy the following to cite this article: Amara-Rekkab A, Didi M. A. Removal of Cd (II) and Hg(II) by chelating resin Chelex-100. Orient J Chem 2015;31(1). |
| Copy the following to cite this URL: Amara-Rekkab A, Didi M. A. Removal of Cd (II) and Hg(II) by chelating resin Chelex-100. Orient J Chem 2015;31(1). Available from: http://www.orientjchem.org/?p=7708 |
Introduction
Heavy metals such as cadmium (II) and mercury (II) are widely distributed throughout the environment as a result of soil erosion and of a broad range of industrial and agricultural processes. Inhalation of cadmium fumes produces emphysema then bronchitis. Mercury poisoning results in severe nausea, vomiting, and abdominal pain; kidney damage is also reported. The determination of low levels of Cd (II) and Hg (II) in environmental samples and inexpensive removal of the species from water are very important [1].
Up to now, many methods have been proposed to determine metal ions, such as atomic absorption spectrometry (AAS), inductively coupled plasma atomic emission spectrometry (ICP-AES), spectrophotometry, high performance liquid chromatography, reversed-phase liquid chromatography, and ion chromatography. The reinto spectrophotometer method is widely used for its simpleness. While the poor special properties of color reagent, which is needed in the spectrophotometer method, make it difficult to determine several metals at the same time because of the absorption spectra overlapping problem [2].
In this paper, a new method for the simultaneous determination of Cd2+ and Hg2+ was developed, using Pyridylazonaphtol (PAN) as chromomeric reagent by spectrophotometry method and their extraction using resin Chelex 100 as extractant agent.
Chelating resins have seen considerable application in speciation studies, particularly the commercially available Chelex-100 resin, which is a polystyrene divinylbenzene copolymer incorporating iminodiacetate chelating groups. The iminodiacetate groups coordinate metals by means of oxygen and nitrogen bonds and the resins have a particularly strong affinity for trace metals. It was proposed firstly to use Chelex-100 for the preconcentration of total trace metals from seawater. After, she is used to differentiate labile from non-labile fractions of trace metals. Chelex-100 retains free metal ions and loosely bound trace metals [3].
Chelex 100 finds application in many fields, it was effective in binding several metal ions (Cr3+, Ni2+, Cu2+and Zn2+, Tl3+, La3+ and Al3+) [4-9].
Therefore, the objectives of this study are to investigate the best performance of mixture of Hg (II) and Cd (II) extraction by Chelex 100, by varying diverse parameters as the initial mixture ion concentration, initial pH of aqueous solution, ion strength and the amounts of resin.
Materials and Methods
Chelex -100 (Bio-Rad Laboratories, CA, USA) is a chelating resin which uses ion exchange to bind transition metal ions. The resin is composed of polystyrene divinylbenzene copolymers containing paired iminodiacetate ions, which act as chelators for polyvalent metal ions (see Table 1) [10]. This group can interact via
its nitrogen and oxygen atoms with the mercury according a tridentate interaction.
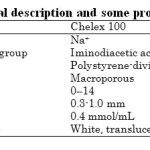 |
Table1: General description and some properties of resin Click here to View table |
Mercury (II) and Cadmium (II) chloride are procured from Sigma (St. Louis, MO, USA). Buffer (5.5, 10.0 and 13) was obtained from Riedel-de-Haen (Seelze, Germany), while sodium chloride is from PANREAC (Phillipsburg, USA). Pyridylazonaphtol PAN (purity >99%) used for mercury analysis were from Sigma (St. Louis, MO, USA). Hydrochloric acid and sodium hydroxide used for adjusting pH of mixture solutions were from Stinnes chemicals (Deutscland), Sigma (St. Louis, MO, USA), respectively.
The concentration of PAN stock solution ranged from 10-3 mol.L-1. Typically, the concentration of mixture of mercury (II) and cadmium (II) was 10-3mol.L-1, in a representative experiment, the quartz cellule contained different volumes of the PAN solution (50- 250 μL) and 700 μL of the different pH buffer (5.5, 10.0 and 13.0). Aliquots of (50- 250 μL) of mixture solution were added and the absorbance of PAN and its complexes were recorded after each addition.
Kinetic experiments were carried out by agitating 5.0 mL of mixture of Hg (II) and Cd (II) solution at initial concentration, 1 mmol /L, with 0.1 g of chelating resin Chelex 100 in a 10 mL Erlen at 20±1 °C at pH=4.75 and at a constant agitation speed of 1000 rounds per minute (rpm) for a time ranging from 2 to 180 min and the amount of mixture remaining in solution was measured. All experiments were made at pH = 4.75 without adjustment. The samples were collected from the shaker, filtered and the filtrates were analyzed for mixture of Hg (II) and Cd (II) concentrations with a SPECORD 210 plus spectrophotometer at 590 nm using Pyridylazonaphtol (PAN) and buffer at pH=13.0 as reagents.
The percent of mixture of Hg (II) and Cd (II) extraction, (%) was determined as follows:
Yield (%) = 100. (C0-C)/C0 (1)
The adsorption amount was calculated as follows:
qt = V(C0-C) M/W (2)
where qt is the adsorption amount (mg/g), w the weight of the Chelex 100 (g), M molar mass (g/mol), V the volume of solution (L), and C0 and C are the concentrations (mol/L) of mixture ions before and after adsorption, respectively.
The effect of solution pH on the equilibrium uptake of mixture of Hg (II) and Cd (II) from aqueous solution by the Chelex 100 resin investigated between pH 1.6 and 10.0. The experiments were performed by adding a known weight of resin (0.1 g) into eight 10 mL Erlen containing 5 mL of mercury (II) and cadmium (II) solution. Dilute nitric acid or sodium hydroxide was used to adjust the pH of mixture solutions using a pH meter (model WTW, PH 3310 SET 2, Germany). The flasks were shaken at 1000 rpm at 20±1 °C for 20 min.
Kinetic experiments were carried out by agitating 5.0 mL of mixture solution of concentration ranging from 0.01 to 1 mmol /L with 0.1 g of Chelex 100 resin in a Erlenfrom 10 mL at 20±1 °C, at pH=4.75 and at a constant agitation speed of 1000 rpm for 20 min.
The effect of the adsorbent amount was studied with a 5 mL solution of 1 mmol/L mercury (II) and cadmium (II) solutions and varying amounts of adsorbent from 0.05 to 0.2 mg.
The effect of ionic strength of aqueous media on the equilibrium uptake of mixture of Hg (II) and Cd (II) from the aqueous solution by the Chelex 100 resin (0.1 g) was investigated by adding, in a 10 mL Erlen, a known weight of solid NaCl to 5 mL of 1 mmol/L mixture solution at 20±1 °C at pH=4.75 and at a constant agitation speed of 1000 rpm for 20 min.
Results and Discussion
In an attempt to express the mechanism of mixture of Hg (II) and Cd (II) adsorption onto the surface and pores of the resin, the following kinetic model equations are used to analyze the adsorption experimental data for determination of the related kinetic parameters.
The Pseudo- first order model rate expression based on solid capacity is the most widely used rate equation for assigning the adsorption rate of an adsorbate from a liquid phase and is known as the Lagergren rate equation [11]. It is represented as:
dqt/dt = kf(qe –qt) (3)
where qe (mg/g) and qt (mg/g) are the adsorption capacity at equilibrium and time t respectively and kf (min−1) is the rate constant of the PFO adsorption reaction. On integration and applying boundary conditions as qt= 0 at t = 0 and qt = qe at t = te, Eq. (3) becomes:
Log (qe –qt) = Log qe –kf t/2.303 (4)
The Pseudo- second order model kinetic expression was developed by Ho [12] to describe the adsorption of metal ions onto adsorbent. The rate expression is represented as:
dqt/dt = ks (qe –qt)2 (5)
where qe and qt (mg/g) are the adsorption capacities at equilibrium and time t respectively and ks (g/ mg.min) is the rate constant for the PSO adsorption reaction.
The nonlinear form of PSO model (Eq. (5)) can be rearranged into four different linear forms, of which the most popular one is [13]:

The product k2q2e (mg/g.min) is the initial sorption rate (h).
Applying Eq. (6) for the analysis of kinetic data is usually based on the plotting of t/ qt versus t which should give a linear relationship; whereas, 1/qe and 1/k2qe2 are the slope and the intercept of obtained line, respectively.
Boyd et al. relationship represents an intra-particle diffusion model as follows [14]:
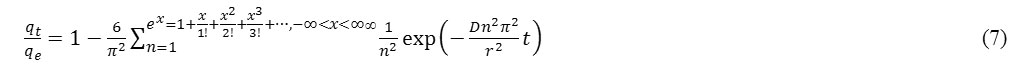
where D is the intra-particle diffusion coefficient and r is the particle radius. For short times (when qt /qe is less than 0.3), Eq. (7) can reduce to the following expression
 where kID is the intra-particle diffusion constant. The significant property of this equation is that, if the intra-particle diffusion is the only rate-limiting step, then the linear plot of qt versus √t should pass through the origin. On the other hand, if the intercept of plots do not equal zero, then it indicates that the intra-particle diffusion is not the sole rate determining step [13]. Then Eq. (8) [15] is modified to:
where kID is the intra-particle diffusion constant. The significant property of this equation is that, if the intra-particle diffusion is the only rate-limiting step, then the linear plot of qt versus √t should pass through the origin. On the other hand, if the intercept of plots do not equal zero, then it indicates that the intra-particle diffusion is not the sole rate determining step [13]. Then Eq. (8) [15] is modified to:

where S is a constant and reflects the boundary layer effect. Investigation of various reports about the sorption rate shows that the intra-particle diffusion model is the most popular one for the diffusion rate-controlling step that has been used in conjunction with the surface reaction models to recognize the adsorption kinetics.
Mole-ratio titrations were performed at pH 5.5, 10.0 and 13.0 to determine the stoichiometry of the Hg (II) – Cd (II) – PAN complexes that form. These pH values were chosen since different protonation states of the 1: 2 complexes dominate under these pH conditions.
![Figure. 1. Spectra from the mole-ratio titration of Hg (II) and Cd (II) into PAN solutions at pH 5.5, [PAN] = ([Hg2+-Cd2+] = 10-3 mol/L, mole ratio ([Hg2+-Cd2+]/[PAN]) of the different spectra: 1:5, 2:1, 1:1, 1:2, 5:1.](http://www.orientjchem.org/wp-content/uploads/2015/03/Vol31_No1_Remo_Afaf_Fig1-150x150.jpg) |
Figure1: Spectra from the mole-ratio titration of Hg (II) and Cd (II) into PAN solutions at pH 5.5, [PAN] = ([Hg2+-Cd2+] = 10-3 mol/L, mole ratio ([Hg2+-Cd2+]/[PAN]) of the different spectra: 1:5, 2:1, 1:1, 1:2, 5:1. Click here to View figure |
![Figure. 2. Spectra from the mole-ratio titration of Hg (II) and Cd (II) into PAN solutions at pH 10.0, [PAN] = ([Hg2+-Cd2+] = 10-3 mol/L, mole ratio ([Hg2+-Cd2+]/[PAN]) of the different spectra: 1:5, 2:1, 1:1, 1:2, 5:1.](http://www.orientjchem.org/wp-content/uploads/2015/03/Vol31_No1_Remo_Afaf_Fig2-150x150.jpg) |
Figure2: Spectra from the mole-ratio titration of Hg (II) and Cd (II) into PAN solutions at pH 10.0, [PAN] = ([Hg2+-Cd2+] = 10-3 mol/L, mole ratio ([Hg2+-Cd2+]/[PAN]) of the different spectra: 1:5, 2:1, 1:1, 1:2, 5:1. Click here to View figure |
![Figure. 3. Spectra from the mole-ratio titration of Hg (II) and Cd (II) into PAN solutions at pH 5.5, [PAN] = ([Hg2+-Cd2+] = 10-3 mol/L, mole ratio ([Hg2+-Cd2+]/[PAN]) of the different spectra: 1:5, 2:1, 1:1, 1:2, 5:1.](http://www.orientjchem.org/wp-content/uploads/2015/03/Vol31_No1_Remo_Afaf_Fig3-150x150.jpg) |
Figure3: Spectra from the mole-ratio titration of Hg (II) and Cd (II) into PAN solutions at pH 5.5, [PAN] = ([Hg2+-Cd2+] = 10-3 mol/L, mole ratio ([Hg2+-Cd2+]/[PAN]) of the different spectra: 1:5, 2:1, 1:1, 1:2, 5:1. Click here to View figure |
Figures 1, 2 and 3 shows the spectral changes that occur with the mole-ratio titrations at pH 5.5, 10.0 and 13.0 with all the titrations, clear isobestic points were obtained which suggests the formation of only one complex.
With a further increase in pH the peak disappear to form a large broad band with a maximum at 591.1 nm.
With increase in pH, complexation occurs with complex peaks arising at 590. The 590.1 nm peak reaches its maximum at pH 13 (Figure 3). At this pH the 591.1 nm peak is higher than the 591.1 nm peak as opposed to the spectrum under acidic conditions (Figure 1). It therefore appears that complexation occurs under these relatively high pH conditions.
The results from these titrations indicate that the mixture of mercury and cadmium only forms 1:2 complexes with PAN in the pH range 13.0±1.0.
The influence of contact time on the percent [Hg – Cd] (II) extraction (%) (Eq. (1)) and the uptake (mg/g) (Eq. (2)) from aqueous solution of mixture, 1 mmol/L, was investigated at 20±1 °C (Figure. 4).
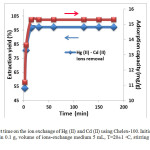 |
Figure4: Effect of contact time on the ion exchange of Hg (II) and Cd (II) using Chelex-100. Initial concentration of mixture 1 mmol/L, amount of resin 0.1 g, volume of ions-exchange medium 5 mL, T=20±1 ◦C, stirring time 1000 rpm and initial pH=4.75. Click here to View figure |
It is essential to evaluate the effect of contact time on the adsorption prior to the kinetic study of the adsorption. The experiments are conducted when the contact time varies from 2 to 180 min, and the results obtained are shown in Figure 4. It can be seen that the adsorption percentage increases considerably until the contact time reaches 15 min. Further increase in contact time does not enhance the adsorption percentage obviously. The phenomenon may be due to the fact that, initially, all active sites on the adsorbents surface are vacant and the solution concentration is high. After that period, few surface active sites are available, so only a very low increase in the metal uptake is observed. Therefore, a contact time of 20 min is selected for all the equilibrium tests.
In this study, batch sorption kinetics of Hg (II) and Cd (II) ions at initial concentration, 1 mmol /L, with the chelating resin Chelex 100 has been studied. The different values of constants from the slopes and intercepts of linear plots of Eq. (4) (shown in Figure 5), Eq. (6) (shown in Figure 6), are summarized in Table 2.
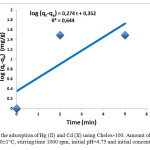 |
Figure5: Lagergren plots for the adsorption of Hg (II) and Cd (II) using Chelex-100. Amount of resin 0.1 g, volume of ions-exchange medium 5mL, T= 20±1°C, stirring time 1000 rpm, initial pH=4.75 and initial concentration of mixture 1 mmol/L.
|
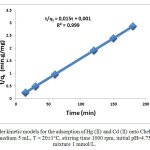 |
Figure6: Pseudo-second order kinetic models for the adsorption of Hg (II) and Cd (II) onto Chelex 100. Amount of resin 0.1 g, volume of ions-exchange medium 5 mL, T = 20±1°C, stirring time 1000 rpm, initial pH=4.75 and initial concentration of mixture 1 mmol/L.
|
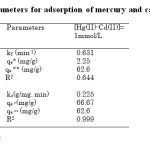 |
Table2: Kinetic parameters for adsorption of mercury and cadmium by Chelex 100 Click here to View table |
As shown in Table 2, the obtained coefficients values of the pseudo-second-order model ( > 0.999) were better than those of the first-order model for the adsorption of Hg (II) and Cd (II) at the considered concentration, suggesting that the pseudo-second-order model was more suitable to describe the adsorption kinetics of Chelex 100 for Hg (II) and Cd (II).This suggests that the rate limiting step may be a chemical process involving valence forces through sharing or exchange of electrons [16, 17].
The mixture of mercury (II) and cadmium (II) ions transport from the solution phase to the surface of the Chelex 100 occurs in several steps. The overall adsorption process may be controlled either by one or more steps (e.g., film or external diffusion, pore diffusion, surface diffusion and adsorption on the pore surface). Besides adsorption at the outer surface of the resin, there is also a possibility of intra-particle diffusion of Hg (II) and Cd (II) from the bulk of outer surface into the pores adsorbent. The possibility of intra-particle diffusion was studied using the Boyd equation (Eq. (9)).
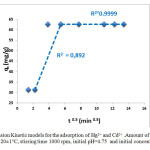 |
Figure7: Intra-particle diffusion Kinetic models for the adsorption of Hg2+ and Cd2+ .Amount of resin 0.1 g, volume of ions-exchange medium 5 mL, T= 20±1°C, stirring time 1000 rpm, initial pH=4.75 and initial concentration of Hg (II) 1 mmol/L.
|
The plot of the Boydrelationship for the sorption of mixture, at initial concentration equal to 1 mmol/L, by the resin is shown in Figure. 7. Therefore, from Figure. 7 it follows that the intra-particle diffusion (at the later portion) proceeds faster than the film diffusion (at the beginning portion). Moreover, the second stage of the lines does not pass through the origin. This means that the intra-particle diffusion, although important over longer contact time periods, is note the rate-limiting step in the adsorption process. The intra-particle diffusion constants and regression coefficients for these two stages (k and R2) are given in Table 3.
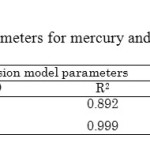 |
Table3: Intra-particle diffusion model parameters for mercury and cadmium adsorption onto Chelex 100 Click here to View table |
The percentage uptake is highly dependent upon the initial concentration of the Hg (II) and Cd (II) ions and the resin. The initial of mixture of Hg (II) and Cd (II) concentrations tested were 0.01, 0.1, 0.2, 0.5, 0.8 and 1 mmol/L at an amount of adsorbent of 0.1 g (Figure 8). The data indicates that the initial metal concentration determines the equilibrium concentration, and also determines the uptake rate of metal ion and the kinetic character of the process.
In the case of low concentrations, the ratio of the initial number of moles of mixture of Hg (II) and Cd (II) ions to the available surface area is larger and subsequently, the fractional ion exchange becomes independent of initial concentrations. The rapid metal extraction has significant practical importance, as this will facilitate with the small amount of resins to ensure efficiency and economy.
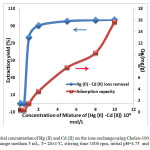 |
Figure8: Effect of initial concentration of Hg (II) and Cd (II) on the ions exchange using Chelex-100. Amount of resin 0.1 g, volume of ions-exchange medium 5 mL, T= 20±1°C, stirring time 1000 rpm, initial pH=4.75 and contact time 20 min.
|
The removal amount of Hg (II) and Cd (II) increased rapidly with increase in the Hg (II) and Cd (II) concentrations. As the mixture of ions concentrations was higher than 5 mmol L−1 it became slow. When the Hg (II) and Cd (II) concentrations was 5 mmol L−1, the amount of Hg (II) and Cd (II) absorbed by the Chelex 100 was 7.73 mg g−1 compared with 0.35 mg g−1 when 0.1 mmol L−1 while the adsorption effective of Hg (II) and Cd (II) kept constant at nearly 100%. At higher concentrations, more Hg (II) was left un-adsorbed in solution due to the saturation of binding sites. The adsorption capacity of mercury and cadmium sorbed on the Chelex 100 after equilibrium is 15.65 mg/g, at an initial concentrations 1 mmol/L.
The pH of solution has been known as the most important variable governing ions adsorption onto adsorbent. This is partly because hydrogen ions themselves are strongly competing with adsorbents [16]. According to the batch method, a series of 5 mL aliquots solution containing a mixture of mercury (II) and cadmium (II) was tested and determined by equilibrating with 0.1 g sorbent at different pH values (range from 1.6 to 10.0). The equilibrium sorption capacity was minimum at pH 1.6 (84.19%) and increased up to pH 2.6, reached maximum (15.65 mg/g) at this pH. This sorption trend can likely be ascribed to the effect of competitive binding between Hg (II) and hydrogen ions for the binding sides on the surface of the resins. At low pH, an excess of hydrogen ions can compete effectively with Hg (II) and Cd (II) for bonding sites, resulting in a lower level of Hg (II) and Cd (II) uptake. This increase is rational for Chelex-100 because the exchanger being weakly acidic, is practically in the hydrogen form at pH 2 and is gradually converted into metal form as the pH was increasing [4].
Figure 9 give, the functional group of the resin is present in four forms depending on the pH. A pHi = 4.75 is the shape (c) predominates.
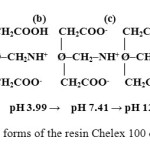 |
Figure9: Different forms of the resin Chelex 100 depending on the pH
|
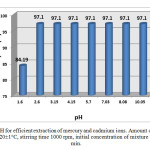 |
Figure10: Effect of initial pH for efficient extraction of mercury and cadmium ions. Amount of resin 0.1 g, volume of ions-exchange medium 5 mL, T= 20±1°C, stirring time 1000 rpm, initial concentration of mixture 1mmol/L and contact time 20 min.
|
Iminodiacetic acid is diprotic in nature and in the resin has one exchange sites in its acetate groups with different selectivity for hydrogen. At initial pH=4.75, the form (b) (Figure 9) was predominant.
The effect of varying doses of the adsorbent Chelex 100 was investigated using 1 mmol/L of mercury (II) and cadmium (II) concentrations. Figure 11 shows an increase in the percentage of extraction of mixture with the increase in dose of the adsorbents up to certain limit (0.125 g of Chelex 100) and then the rate of change in increase becomes negligible.
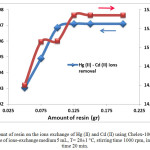 |
Figure11: Effect of amount of resin on the ions exchange of Hg (II) and Cd (II) using Chelex-100. Initial concentration of mixture 1 mmol/L, volume of ions-exchange medium 5 mL, T= 20±1 °C, stirring time 1000 rpm, initial pH=4.75 and contact time 20 min.
|
The increase in the adsorption with increasing doses of adsorbent is likely due to increase in adsorbent surface area and availability of more adsorption sites. The optimum adsorbent dose is found to be 0.125 g of resin.
The effect of ionic strength on mercury (II) and cadmium (II) sorption was studied by stirring 0.1 g of Chelex 100 resin with increasing NaCl amount, in the aqueous solutions, from 0.001 to 0.073 g. Results are summarized in Figure 12 and shows that the influence of the ionic strength on sorption of mixture is not important.
 |
Figure12: Effect of NaCl concentration on the extraction yield. Initial concentration of mixture (II) 1 mmol/L, amount of resin 0.1 g, volume of ions-exchange medium 5 ml, T= 20±1°C, stirring time 1000 rpm, initial pH=4.75 and contact time 20 min.
|
It is evident in Figure. 12, that there is a null impact on increasing of extraction yield of mixture of mercury and cadmium. It seems that the presence of Na+ in solution has a null effect on Hg2+ and Cd2+ adsorptions by Chelex 100.
This investigation has shown that the mixture of mercury (II) and cadmium (II) reacts with PAN in the pH range 1.0±13.0 to form a 1: 2 complex.
Conclusions
A commercial resin the Chelex 100 was tested on mixture of mercury (II) and cadmium (II) extraction. The extraction efficiency was determined as a function of various parameters such as time, pH, mixture concentration, amount of resin and ionic strength effect. The experimental capacity obtained is 15.65 mg/g.
The kinetics of mixture of mercury (II) and cadmium (II) adsorptions on resin follows the pseudo-second order kinetic model and the sorption of Hg (II) and Cd (II) ions was found to be very rapid, value was around 5 min. Kinetics of Hg (II) and Cd (II) adsorptions from the solution on Chelex 100 resin is controlled by the external and film diffusion mechanisms.
The maximum sorption of mercury (II) and cadmium (II) took place in the initial pH up to 2.6 and the mixture concentrations 1mmol/L, the presence of NaCl has a null effect on the extraction efficiency.
References
- E. A. Moawed, Separation and preconcentration of trace amounts of Cadmium (II) and Mercury (II) ions on Rosaniline-grafted polyurethane foam, Acta Chromatogr. 14 (2004)189-214.
- Y. Han, Y. Li, W. Si, D. Wei, Z. Yao, X. Zheng, B. Du, Q. Wei, Simultaneous determination of Cu2+, Zn2+, Cd2+, Hg2+ and Pb2+ by using second-derivative spectrophotometry method, Spectrochimica Acta Part A 79 (2011) 1546– 1551, DOI: 10.1016/j.saa.2011.04.086.
- L.S. Wen, K.T. Jiann, P.H. Santschi, Physicochemical speciation of bioactive trace metals (Cd, Cu, Fe, Ni) in the oligotrophic South China Sea, Mar. Chem. 101 (2006) 104–129, DOI: 10.1016/j. marchem. 2006. 01.005.
- F. Gode, E. Pehlivan, Removal of chromium (III) from aqueous solutions using Lewatit S 100: The effect of pH, time, metal concentration and temperature, J. Hazard. Mater. B 136 (2006) 330–337, DOI: 10.1016/j.jhazmat. 2005.12.021.
- H. Leinonen, J. Lehto, Ion-exchange of nickel by iminodiacetic acid chelating resin Chelex 100, React. Funct. Polym. 43 (2000) 1–6, DOI:10.1016/S1381-5148(98) 000820
- L. C. Lin, R.S. Juang, Ion-exchange kinetics of Cu (II) and Zn (II) from aqueous solutions with two chelating resins, Chem. Eng. J. 132 (2007) 205–213, DOI: 10. 1016 /j.cej.2006.12.019.
- L. Tser-Sheng, J.O. Nriagu, Thallium speciation in river waters with Chelex-100 resin, Anal. Chim. Acta 395 (1999) 301-307, DOI: 10.1016/S0003-2670(99)00358-X.
- R.S.S. Wu, K.H. Lam, J.M.N. Lee, T.C. Lau, Removal of phosphate from water by a highly selective La (III)-Chelex resin, Chemosphere 69 (2007) 289–294, DOI: 10.1016/j.chemosphere.2007.04.022.
- A. Etou, S. Bai, T. Saito, H. Noma, Y. Okaue, T. Yokoyama., Formation conditions and stability of a toxic tridecameric Al polymer under a soil environment, J. Colloid Interface Sci. 337 (2009) 606–609, DOI: 10.1016/j.jcis.2009.05.079.
- P. Kirsty, N. Mc Callum, L. Welch, A comparison of methods for forensic DNA extraction: Chelex-1001 and the QIAGEN DNA Investigator Kit (manual and automated), Forensic. Sci. Int: Genetics 6 (2012) 282–285, DOI: 10.1016/j.fsigen.2011.04.018.
- Y.S. Ho, Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics 59 (2004) 171-177, DOI: 10.1023/B:SCIE. 0000013305. 99473.cf.
- Y.S. Ho, Review of second-order models for adsorption systems, J. Hazard. Mater. B 136 (2006) 681–689, DOI: 10.1016/j.jhazmat. 2005.12.043.
- M. Haerifar, S. Azizian, Mixed Surface Reaction and Diffusion-Controlled Kinetic Model for Adsorption at the Solid/Solution Interface, J. Phys. Chem. C 117 (2013) 8310−8317, DOI: 10.1021/jp401571m.
- G. E. Boyd, A. W. Adamson, L. S. JrMyers, The Exchange Adsorption of Ions from Aqueous Solutions by Organic Zeolites. II. Kinetics. J. Am. Chem. Soc. 69 (1947) 2836−2848.
- P. Barkakati, A. Begum, M. L. Das, Adsorptive separation of Ginsenoside from aqueous solution by polymeric resins: Equilibrium, kinetic and thermodynamic studies, Chem. Eng. J. 161 (2010) 34–45, DOI: 10.1016/j.cej.2010.04.018.
- Y.S. Ho, G. McKay, The kinetics of sorption of divalent metal ions onto sphagnum moss peat, Water Res. 34 (2000), 735–742, DOI: 10.1016/S0043-1354(99) 00232-8.
- D. Li, X. Chang, Z. Hu, Q. Wang, R.Li, X. Chai, Samarium (III) adsorption on bentonite modified with N-(2-hydroxyethyl) ethylene- diamine, Talanta 83 (2011) 1742–1747, DOI: 10.1016/j.Talanta. 2010.12.012.13

This work is licensed under a Creative Commons Attribution 4.0 International License.









