Kinetic and Thermodynamic study for the Oxidation of 4-Oxo-4-phenyl butanoic Acid by Tripropylammonium fluorochromate in Aqueous Acetic Acid Medium
A. Yogananth1 and S. Sheik Mansoor2*
1Research and Development Centre, Bharathiar University, Coimbatore – 641 046, Tamil Nadu, India 2Department of Chemistry, C. Abdul Hakeem College (Autonomous), Melvisharam – 632 509, Tamil Nadu, India
DOI : http://dx.doi.org/10.13005/ojc/310102
Article Received on :
Article Accepted on :
Article Published : 07 Feb 2015
Tripropylammonium fluorochromate (TriPAFC) in acetic acid-water medium oxidizes 4-oxo-4-phenyl butanoic acid (4-Oxo acid) in the presence of perchloric acid. The reaction is first order each in [TriPAFC], [4-Oxo acid] and [H+]. From the observed kinetic results a suitable mechanism has been proposed.
KEYWORDS:4-Oxo acid; kinetics; oxidation; tripropylammonium fluorochromate
Download this article as:| Copy the following to cite this article: Yogananth A, Mansoor S. S. Kinetic and Thermodynamic study for the Oxidation of 4-Oxo-4-phenyl butanoic Acid by Tripropylammonium fluorochromate in Aqueous Acetic Acid Medium. Orient J Chem 2015;31(1). |
| Copy the following to cite this URL: Yogananth A, Mansoor S. S. Kinetic and Thermodynamic study for the Oxidation of 4-Oxo-4-phenyl butanoic Acid by Tripropylammonium fluorochromate in Aqueous Acetic Acid Medium. Orient J Chem 2015;31(1). Available from: http://www.orientjchem.org/?p=7009 |
Introduction
The oxidation of organic substrates is an important aspect in modern organic synthesis, therefore, the search for new oxidizing agents is of interest to synthetic organic chemists. In recent years, there is continued interest in the development of new Cr(VI) reagents for the effective and selective oxidation of organic substrates, under mild conditions, because Cr(VI) is established as a versatile oxidant for the oxidation of a variety of organic compounds1. Many Cr(VI) reagents like benzimidazolium fluorochromate2, tetrahexylammonium fluorochromate3, tributylammonium chlorochromate4, quinolinium chlorochromate5, quinolinium fluorochromate6 and tetraethylammonium chlorochromate7 have been studied in recent years for the oxidation of various organic substrates.
Tripropylammonium fluorochromate is also one such oxidant developed recently8-11. It is a more efficient and stronger oxidizing agent. This new compound is more efficient for quantitative oxidation of several organic substrates and has certain advantages over similar oxidizing agents in terms of the amount of oxidant and solvent required, short reaction times and high yields.
Extensive studies on the mechanism of oxidation of 4-oxo acids by different oxidants have been the subject of study by various workers12–17. However, no detailed kinetic study of oxidation of 4-oxo acids by TriPAFC have so far been attempted. We have been interested in the kinetic and mechanistic aspects of the oxidation by complexed Cr(VI) species, and several studies on halochromates have already been reported18–21. In continuation of our earlier work, we now report the kinetics and mechanism of oxidation of 4-oxo-4-phenyl butanoic acid by TriPAFC in aqueous acetic acid medium.
Experimental
Materials
The 4–oxo–4–phenyl butanic acid is synthesized by the Friedel–Craft’s reaction between succinic anhydride and benzene in the presence of anhydrous aluminium chloride14. Tripropylammonium bromide and chromium trioxide were obtained from Fluka (Buchs, Switzerland). Acetic acid was purified by standard method and the fraction distilling at 118 oC was collected. Perchloric acid is used as the source of hydrogen ion.
Kinetic Measurements
The pseudo – first-order conditions were attained by maintaining a large excess ( x 15 or more) of 4-Oxo acid over TriPAFC. The solvent was 50% acetic acid – 50% water (v/v), unless specified otherwise. The reactions were followed, at constant temperatures (± 0.01 K), by monitoring the decrease in [TriPAFC] spectrophotometrically at 364 nm using UV–Vis spectrophotometer, Shimadzu UV-1800 model. The pseudo-first-order rate constant kobs, was evaluated from the linear (r = 0.990 to 0.999) plots of log [TriPAFC] against time for up to 80% reaction. The second order rate constant k2, was obtained from the relation k2 = kobs / [4-Oxo acid].
Product Analysis
Product study was made under mineral acid catalysed condition in 4–oxo–4–phenyl butanic acid. Keeping the concentration of TriPAFC in excess over 4–oxo–4–phenyl butanic acid, the two solutions were mixed together in 50% acetic acid – 50% water (v/v). The reaction mixture was allowed to go to completion by keeping it in a thermostat at 303 K for 24 h to ensure the completion of the reaction. The product was obtained which had melting point of 121 oC. It was dissolved in benzene and a careful TLC analysis was done with benzoic acid and 4–oxo–4–phenyl butanoic acid as references. Only one spot corresponding to benzoic acid was obtained.
Stoichiometric Studies
The stoichiometric studies for the oxidation of 4-oxo acid by TriPAFC were carried out with oxidant in excess. The solvent composition 50% acetic acid – 50% water (v/v) and [H+] were maintained as in the corresponding rate measurements. The temperature was maintained at 303 K. The 4-oxo acid and TriPAFC were mixed in the ratio 1:4, 1:5, 1:6 and were allowed to react for 24 h at 303 K. The concentration of unreacted TriPAFC was determined. ∆[TriPAFC] was calculated. The stoichiometry was calculated from the ratio between [4-Oxo acid] and [TriPAFC]. The estimation of unreacted TriPAFC showed that 1 mol of TriPAFC reacts with 1 mol of 4–oxo–4–phenyl butanic acid.
Results and Discussion
Tripropylammonium fluorochromate is easily prepared as reported in the literature9. The oxidation 4–oxo–4–phenyl butanic acid by TriPAFC has been conducted in 50% acetic acid and 50% water medium at 303 K, under pseudo first order conditions and the result obtained were discussed in the following paragraphs.
Order of the Reaction
The reactions are first order with respect to TriPAFC as evidenced by a linear plot of log [TriPAFC] against time. The pseudo first-order rate constants do not depend on the initial concentration of TriPAFC (Table 1). This further confirms first-order dependence of rate on oxidant concentration. The concentration of 4-oxo acid is varied in the range of 1.2 x 10-2 to 2.8 x 10-2 mol dm-3 at 303 K and keeping all other reactant concentrations as constant and the rates were measured (Table 1). The data establish that the rate increases with increase in [4-Oxo acid] in a first-order fashion. Further, the plot of k1 versus [4-Oxo acid] is excellently linear passing through origin (Figure 1). Also, the k2 values remain constant when [4-Oxo acid] is varied (Table 1). This result, coupled with the slope value of 0.98 for the double logarithmic plot between kobs and [4-Oxo acid] (Figure 2) confirms the first-order nature of the reaction with respect to [4-oxo acid]. The acid catalysed nature of this oxidation is confirmed by an increase in the rate on the addition of H+. The plot of log k1 versus log [H+] is a straight line with the slope of 0.996 (Fig. 3). Therefore, order with respect to [H+] is one. TriPAFC may become protonated in the presence of acid and the protonated TriPAFC may function as an effective oxidant.
Effect of Acidity
The reaction is catalyzed by hydrogen ions (Table – 1). The acid–catalysis may well be attributed to a protonation of TriPAFC to give a stronger oxidant and electrophile.
![]()
The formation of a protonated Cr(VI) species has earlier been postulated in the reactions of structurally similar PFC22.
Effect of Acrylonitrile and Mnso4
The reaction did not promote polymerization of acrylonitrile indicating the absence of free radicals (Table – 1). However, the addition of Mn(II) (0.003 mol dm–3), in the form of MnSO4 retards the rate of oxidation. This indicates the involvement of Cr(IV) intermediate in the oxidation of 4-oxo acid by Cr(VI) reagent and confirms the two electron transfer process in the reaction. Mn(II) ion reduces Cr(IV) formed to Cr(III). In the absence of Mn(II) ion, formed Cr(IV) reduces Cr(VI) to Cr(V) and the oxidation of 4-oxo acid by Cr(V) is fast23. The decrease in the rate of Cr(VI) reduction on the addition of Mn(II) has been attributed to the removal of Cr(IV) by reaction24 with Mn(II).
 |
Table1: Rate constants for the oxidation of 4-oxo-4-phenyl butanoic acid by TriPAFC in aqueous acetic acid medium at 303 Ka Click here to View table |
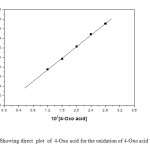 |
Figure1: Showing direct plot of 4-Oxo acid for the oxidation of 4-Oxo acid by TriPAFC Click here to View figure |
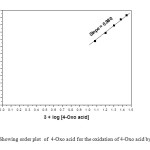 |
Figure2: Showing order plot of 4-Oxo acid for the oxidation of 4-Oxo acid by TriPAFC Click here to View figure |
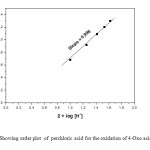 |
Figure3: Showing order plot of perchloric acid for the oxidation of 4-Oxo acid by TriPAFC Click here to View figure |
Effect of Solvent Polarity on Reaction Rate
The oxidation of 4-oxo-4-phenyl butanoic acid has been studied in the binary mixture of acetic acid and water as the solvent medium. For the oxidation of 4-oxo-4-phenyl butanoic acid, the reaction rate increased remarkably with the increase in the proportion of acetic acid in the solvent medium. These results are presented in Table 2. The plots of log k1against the inverse of the dielectric constant are linear with positive slopes, indicating an interaction between a positive ion and dipolar molecule (Fig 4). Amis (1967) holds the view that in an ion–dipole reaction involving a positive ionic reactant, the rate would decrease with increasing dielectric constant of the medium and if the reactant were to be a negatively charged ion, the rate would increase with the increasing dielectric constant25.
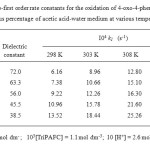 |
Table2: Pseudo-first order rate constants for the oxidation of 4-oxo-4-phenyl butanoic acid by TriPAFC at various percentage of acetic acid-water medium at various temperatures Click here to View table |
In the present study, the significant rate enhancement with the lowering of dielectric constant of the solvent medium may be attributed to the enolisation of the ketoacid group in the 4-oxo-4-phenyl butanoic acid. The enolisation of the keto group of the oxoacid is facilitated by the increase in percentage of acetic acid in the solvent medium and this may also favors the rate enhancement.
Rate of Enolisation by Bromination Method
It has been reported earlier in the case of oxidation of keto compounds that the oxidation proceeds via enolisation of the keto compounds. The rate of enolisation of keto compound is faster than the rate of oxidation. The reactive species of the substrate may be determined by enolisation, which is an acid as well as base catalysed reaction and proceeds by a concerted or push–pull mechanism. The rate of enolisation was determined by bromination method for the system under investigation.
The order of bromination reaction with respect to the 4-oxo-4-phenyl butanoic acid, bromine and H+ has been determined. These data indicate that the bromination of the 4-oxo-4-phenyl butanoic acid is first order each with respect to the substrate and H+ ion but zero order with respect to bromine.
Thermodynamic Parameters
The kinetics of oxidation of 4-oxo acid is studied at four different temperatures viz., 298, 303, 308 and 313 K at various percentage of acetic acid-water medium. The second order rate constants were calculated (Table 3). The Arrhenius plot of log k2 versus 1/T is found to be linear. The enthalpy of activation, entropy of activation and free energy of activation were calculated from k2 at 298, 303, 308 and 313 K using the Eyring relationship by the method of least square and presented in Table 3. The least square method gives the values and standard errors of enthalpy and entropy of activation respectively. Statistical analysis of the Eyring equation clearly confirms that the standard errors of ∆H# and ∆S# correlate26. The entropy of activation is negative for 4-oxo acid. The negative entropy of activation in conjunction with other experimental data supports the mechanism of oxidation.
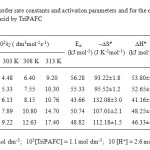 |
Table3: Second order rate constants and activation parameters and for the oxidation of 4-oxo-4-phenyl butanoic acid by TriPAFC Click here to View table |
 |
Figure4: Plot of 1 / D against log k1 showing effect of solvent polarity at various temperatures Click here to View figure |
Mechanism of Oxidation
A probable mechanism for the oxidation of 4-oxo-4-phenyl butanoic acid by TriPAFC has been proposed based on the experimental results and in analogy with the oxidation of oxo compounds with the other oxidants. The results obtained in the kinetic study are briefly summarized below: The course of oxidation does not involve any free radical intermediate. The reaction is first order each in [TriPAFC], [4-Oxo acid] and [H+]. The linear increase in the reaction rate with the increase in [H+] ion is attributed to the formation of protonated TriPAFC i.e. TriPAFCH+ and to the enolisation of the 4-oxo-4-phenyl butanoic acid. The formation of TriPAFCH+ ion and the enolisation of the 4-oxo-4-phenyl butanoic acid is facilitated at lower dielectric constant of the medium. The reaction rate is increases markedly with the increase in the proportion of acetic acid in the medium. When the acid content increases in the medium, the acidity of the medium is increased whereas the dielectric constant of the medium is decreased. The rate of enolisation is found to be greater than the rate of oxidation. Considering these facts and findings a suitable mechanism has been proposed for the oxidation (Scheme–1).
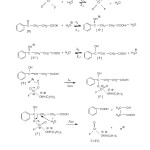 |
Scheme1: Mechanism of oxidation of 4-Oxo acid Click here to View Scheme |
Conclusions
The kinetics of oxidation of 4-Oxo acid by TriPAFC was studied in 50% acetic acid – 50% water (v/v) at 303 K in the presence of perchloric acid. The stoichiometry study showed that 1 mol of TriPAFC reacts with 1 mol of 4–oxo–4–phenyl butanic acid. The oxidation of 4-oxo acid, in an atmosphere of nitrogen, failed to induce the polymerization of acrylonitrile. The reaction is first order each in [TriPAFC], [4-oxo acid] and [H+]. The rate of enolisation was determined by bromination method for the system under investigation.These data indicate that the bromination of the 4-oxo-4-phenyl butanoic acid is first order each with respect to the substrate and H+ ion but zero order with respect to bromine. Considering these facts and findings a suitable mechanism has been proposed for the oxidation.
References
- Patel, S.; Mishra, B. K. Tetrahedron, 2007, 63, 4367-4406.
- Murugesan, V.; Sivamurugan, V.; Rajkumar, G. A.; Arabindoo, B. Indian J. Chem. 2005, 44A, 144-147.
- Koohestani, B.; Javanshir, Z.; Ghammamy, S.; Mehrani, K.; Afrand, H.; Saghatforoush, L. J. Mex. Chem. Soc. 2008, 52, 116–119.
- Mansoor, S. S.; Shafi, S. S. React. Kinet. Mech. Catal. 2010, 100, 21–30.
- Rai, K. K.; Kannaujia, R. K.; Rai, K.; Singh, S. Orient. J. Chem. 2013, 29(3), 1071–1078.
- Bhuvaneshwari, D. S.; Elango, K. P. J. Serb. Chem. Soc. 2008, 73 (7), 735–744.
- Swami, P.; Yajurvedi, D.; Mishra, P.; Sharma, P. K. Int. J. Chem. Kinet. 2010, 42, 50-55.
- Ghammamy, S.; Sharifnezhad, Z.; Aghbolagh, Z. S.; Sahebalzamani, H. Adv. Appl. Sci. Res., 2010, 1(2), 119-123.
- Ghammamy, S.; Khorsandtabar, S.; Moghimi, A.; Sahebalzamani, H. J. Mex. Chem. Soc. 2009, 53(2), 41-43.
- Mansoor, S. S.; Shafi, S. S. J. Mole. Liq. 2010, 155, 85-90.
- Mansoor, S. S. E – J. Chem. 2011, 8, 643-648.
- Sikkandar, G.; Ahmed, K. A. B. Indian J. Chem. 1999, 38A, 183-186.
- Kavitha, S.; Pandurangan, A.; Alphonse, I. Indian J. Chem. 2005, 44A, 715-718.
- Farook, N. A. M. J. Iranian Chem. Soc. 2006, 3, 378-386.
- Reddy, C. S.; Manjari, P. S. Indian J. Chem. 2010, 49A, 418-424.
- Farook, N. A. M.; Dameem, G. A. S. E-J. Chem. 2011, 8, 561-564.
- Farook, N. A. M.; Manochitra, S.; Banu, A. A. J. Solution Chem. 2013, 42, 239-250.
- Asghar, B. H.; Malik, V. S.; Mansoor, S. S. Arab. J. Chem. 2014, doi: org/10.1016/j.arabjc.2014.10.047
- Mansoor, S. S.; Shafi, S. S. Arab. J. Chem. 2014, 7, 171-176.
- Mansoor, S. S.; Shafi, S. S. Arab. J. Chem. 2014, 7, 312–318.
- Mansoor, S. S.; Shafi, S. S. Arab. J. Chem. 2011, doi:10.1016/j.arabjc.2011.01.031
- Sharma, V.; Sharma, P. K.; Banerji, K.K. J. Chem. Research(S). 1996, 290-291.
- Karunakaran, C.; Suesh, S. J. Phys. Org. Chem. 2004, 17, 88-93.
- Khan, Z.; Dar, M. Y.; Babe, P. S. B. Indian J. Chem. 2004, 42A, 1060-1065.
- E. S. Amis, Solvent Effects on Reaction Rates and Mechanisms. Academic Press, New York, 1967, 42.
- Lente, G.; Fabian, I.; Poe, A. J. New J. Chem. 2005, 29, 759-760.

This work is licensed under a Creative Commons Attribution 4.0 International License.









