Inhibition of Mild Steel Corrosion in 1M H2SO4 Medium by Benzimidazole Mannich bases
P. M. Dasami1, K. Parameswari*2, S. Chitra2
1Department of chemistry, PSGR Krishnammalcollege for women, Peelamedu, Coimbatore-641004, Tamil Nadu, India.
2Department of chemistry, PSGR Krishnammal college for women, Peelamedu, Coimbatore- 641 004, Tamil Nadu, India,
DOI : http://dx.doi.org/10.13005/ojc/310120
Article Received on :
Article Accepted on :
Article Published : 21 Mar 2015
The corrosion inhibition behaviour of two synthesized Mannich bases has been investigated for mild steel in 1M H2SO4 by weight loss and electrochemical techniques.The inhibition efficiency depends on concentration of the inhibitor and temperature. The inhibitors function by adsorption on mild steel surface and obey Langmuir isotherm indicating monolayer adsorption on the surface. Thermodynamic parameters show that the adsorption of the inhibitors occurs through electrostatic interaction. Polarization studies reveal that the inhibitors behave as mixed type in 1M H2SO4 affecting both anodic metal dissolution and cathodic hydrogen evolution. SEM studies show the formation of surface adsorptive film of the Mannich bases on the mild steel surface.
KEYWORDS:Inhibitors; Mannich bases; Potentiodynamic polarization; Langmuir adsorption isotherm; Electrochemical impedance
Download this article as:| Copy the following to cite this article: Dasami P. M, Parameswari K, Chitra S. Inhibition of Mild Steel Corrosion in 1M H2SO4 Medium by Benzimidazole Mannich bases. Orient J Chem 2015;31(1). |
| Copy the following to cite this URL: Dasami P. M, Parameswari K, Chitra S. Inhibition of Mild Steel Corrosion in 1M H2SO4 Medium by Benzimidazole Mannich bases. Orient J Chem 2015;31(1). Available from: http://www.orientjchem.org/?p=8003 |
Introduction
Mild steel is largely used in industries as structural material, which undergoes corrosion easily in acidic medium. Acids are widely used for various industrial processes such as pickling, cleaning, descaling, acidizing etc. Because of the aggressiveness of the acids, inhibitors are used to reduce the rate of dissolution of mild steel. Compounds containing nitrogen, sulphur and oxygen have been reported as excellent inhibitors (1,2). Nitrogen and sulphur atoms in the molecules are the active centers for the process of adsorption of the compounds on the metal surface to form a protective layer(3).Mannich bases are pharmaceutically important compounds and reported to show a wide range of bioactivities. They are also used in polymer chemistry especially as paints and surface active agents. Though Mannich bases are known for many decades, it was only recently that they have been used as corrosion inhibitors. The versatile utility of Mannich bases and their complexation characteristics with many transition metal ions prompted us to prepared two new Mannich bases and evaluate their corrosion inhibition performance for mild steel in 1M H2SO4. The investigation is carried out using electrochemical and gravimetric measurements(4).
Methodology
Chemical composition of the mild steel
The experiments were performed with cold rolled mild steel specimen of chemical composition (C=0.20%,Mn=1%,Si-0.05%,S=0.025%,P=0.25% and Fe=98%).
Structure of the Mannich Bases
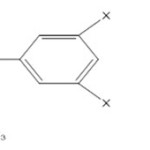 |
Scheme 1 Click here to View scheme |
5-(1-((dimethylamino/dimethylnitro)methyl)-1H-benzo[d]imidazol-2-yl)benzene-1,3-diamineThe structure was confirmed by FTIR Spectra. (KBr) υ cm-1: 1215.21(C-N str.), 1572.05 (C=C str.), 1678.11 (C=N), 1281.75 (C-H bend.), 3603.18 (NH2)
 |
Figure1: FTIR Spectrum of MB1Click here to View figure |
Corrosion Monitoring Methods
Weight loss measurements were carried out with rectangular mild steel plates of dimension 3cm× 1cm× 0.1cm. The polished, preweighed plates were immersed in 1M H2SO4 containing different concentrations of the inhibitors for 3 hrs and then reweighed. From the loss in mass, inhibition efficiency was calculated.
The electrochemical studies were performed on IVIUM Compact stat(potentiostat/galvanostat), using mild steel rod with exposed area 0.785 cm2. The potential range is -200 to +200 mV with respect to the open circuit potential at a scan rate of 1mV/sec. For impedance measurements, the sweep frequency is from 10 Hz to 0.01 Hz with a signal amplitude of 10 mV. SEM micrographs of the mild steel immersed in 1M H2SO4 with and without inhibitor and the morphology was compared.
Results and Discussions
Weight Loss Measurements
The value of percentage inhibition efficiency (IE%) obtained from weight loss method at different concentrations of the inhibitors in 1 M H2SO4 at room temperature are summarized in Table (1). The values show that both the inhibitors MB 1& MB 2 inhibit the corrosion of mild steel in H2SO4 solution,at all concentrations used. Maximum inhibition efficiency was shown at 0.5 mM concentration of the inhibitor.The behavior may be attributed to an increase in surface coverage, Ѳ by the adsorption of inhibitors on the mild steel surface, in the aggressive solution, which restricts the dissolution of the metal.
Table: 1. Inhibition efficiencies of the inhibitors at various concentrations for mild steel corrosion in 1M H2SO4 at 30±1º C
| Concentration, mM | Inhibition Efficiency,% | |
| MB 1 | MB 2 | |
| 0.1 | 57.55 | 56.38 |
| 0.2 | 65.55 | 64.03 |
| 0.3 | 79.68 | 71.92 |
| 0.4 | 80.58 | 80.15 |
| 0.5 | 83.61 | 82.40 |
Influence of Temperature
In order to study the effect of temperature on corrosion inhibition of mild steel in acid and to determine the activation energy for the corrosion process, the weight loss studies were carried out at higher temperatures(303-333 K). Analysis of the results in Table-2 show that the inhibition efficiency decreased with increase in temperature. This may be attributed to the desorption of the inhibitor molecule from metal surface at higher temperature indicating that the inhibitors get adsorbed predominantly by physisorption on the mild steel surface (5).
Table 2: inhibition efficiency at 0.5mM concentration of the inhibitors for mild steel corrosion in 1M H2SO4 at various temperatures
| Name of the compound | Temperature ,K | Weight loss,g | Inhibition efficiency,(%) | Corrosion rate,mpy |
| Blank | 303 | 0.0609 | – | 76627.43 |
| 313 | 0.1152 | – | 144950.4 | |
| 323 | 0.1443 | – | 181565.5 | |
| 333 | 0.2486 | – | 312801 | |
| MB 1 | 303 | 0.0422 | 83.61 | 53,101.64 |
| 313 | 0.0518 | 79.88 | 65,181.64 | |
| 323 | 0.0575 | 77.66 | 72,354.14 | |
| 333 | 0.0622 | 75.84 | 78,268.30 | |
| MB 2 | 303 | 0.0453 | 82.40 | 57,002.48 |
| 313 | 0.0566 | 78.01 | 71,221.64 | |
| 323 | 0.0673 | 73.86 | 84,685.80 | |
| 333 | 0.0743 | 71.14 | 93,494.13 |
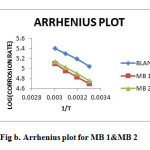 |
Figure2:. Arrhenius plot for MB 1&MB 2 Click here to View figure |
 |
Figure3: Transition State plot for MB 1& MB2 Click here to View figure |
Thermodynamic Parameters
Thermodynamic parameters such as Ea, ∆G,ΔH and ΔS were calculated using Arrhenius plot(Fig.b) and Transition state plot(Fig.c). Ea value is greater in the presence of inhibitor than that in blank acid. The increase in Ea in the presence of inhibitor is typical of physisorption(6). Negative ∆G0ads values indicate spontaneity of the adsorption process and the values up to 40 kJ/mol suggests electrostatic interactions between the charged molecules of the inhibitor and the charged metal surface (i.e.,physisorption)(7). The negative value of ΔH0 reflects the exothermic nature of dissolution process. The value of entropy change ΔS0 in the presence of inhibitor is negative, which implies that the activated complex represents an association rather than dissociation.
Table: 3 Thermodynamic Parameters for mild steel corrosion in 1M H2SO4
| Name of the inhibitor in mM | Activation energy, Ea (kJ/mole) | -ΔG0ads(kJ/mole) | -∆H0 kJ/mole | -∆S0 kJ/mole | |||
| 303 K | 313 K | 323K | 333 K | ||||
| Blank | 22.695 | – | – | – | – | – | – |
| MB 1 | 25.666 | 14.67 | 14.61 | 14.81 | 15.05 | 62.43 | 0.9988 |
| MB 2 | 24.595 | 14.49 | 14.39 | 14.39 | 14.56 | 58.87 | 0.9966 |
Langmuir Adsorption Isotherm
Basic information on the interaction between the surface of mild steel and inhibitor can be determined by studying adsorptionisotherms. The commonly used adsorption isotherms are the Langmuir, Temkin, Frumkin, and Flory–Huggins. The degree of surface coverage(θ) for different concentrations of the inhibitors have been evaluated. The data were tested graphically to determine a suitable isotherm. A straight line with correlation coefficient (R2) almost equal to 1.0, was obtained on plotting Cinh/θ versus Cinh is shown in Fig. d, indicating that adsorption of the inhibitors on the mild steel surface obeys the Langmuir adsorption isotherm. It is an ideal isotherm for physical or chemical adsorption where there is no interaction between the adsorbed molecules(8). According to Lang muiradsorption isotherm, the surface coverage (θ) is related to inhibitor concentration (Cinh) by equation (1) (9):
C/Ѳ = 1/K + C………………………..(1)
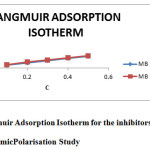 |
Figure4: Langmuir Adsorption Isotherm for the inhibitors MB 1 & MB 2 Click here to View figure |
Where ‘K’ is the equilibrium constant of adsorption.The slopes of straight lines (Fig.d) were found to be very close to unity. This means that, adsorbed molecule occupies only one site and it does not interact with other adsorbed species (10)
Potentiodynamic Polarisation Study
Potentiodynamic polarization curves for the corrosion of mild steel in 1M H2SO4 in the absence and presence of different concentrations of MB 1 at room temperature is shown in Fig.f.The corrosion current density (Icorr), Tafel slopes [anodic (ba) and cathodic (bc)] were calculated by extrapolation of linear parts of anodic and cathodic curves to the corresponding corrosion potential (Ecorr). The percentage inhibition efficiency (IE %) and the degree of surface coverage (θ) were calculated.
The electrochemical parameters, determined from Tafel polarization curves are summarized in Table 4. The data showed that the current density decreased in the presence of inhibitors than in its absence. The(%) inhibition efficiency increased with increasing inhibitor concentrations. This indicates that the inhibitor molecules are adsorbed on the metal surface. The inhibition was more pronounced with increasing inhibitor concentration. Tafel lines are shifted to both negative and positive potentials with respect to the blank curve by increasing the concentration of the inhibitor,also there only slight changes in Ecorr value.The Icorr value decreases when the concentration of the inhibitor increases.This behavior indicates that both anodic metal dissolution and cathodic hydrogen evolution mechanism are affected in the presence of the inhibitor and inhibitors act as mixed-type.
Table 4: Corrosion parameters for mild steel with selected concentration of the inhibitors in 1M H2SO4 by potentiodynamic polarization method
| Name of the compound | Concentration,mM | Tafel slope, mV/decade | -Ecorr, mV | Icorr(µF/cm2) | Inhibition Efficiency(%) | |
| ba | bc | |||||
| Blank | 69 | 113 | 492.1 | 287.7 | ||
| MB 1 | 0.05 | 61 | 143 | 495 | 55.43 | 80.74 |
| 0.3 | 62 | 155 | 491.8 | 53.95 | 81.26 | |
| 0.5 | 55 | 154 | 478.1 | 42.05 | 85.40 | |
| MB 2 | 0.05 | 59 | 146 | 493.2 | 44.79 | 84.46 |
| 0.3 | 56 | 156 | 486.8 | 37.33 | 87.03 | |
| 0.5 | 51 | 146 | 478.1 | 27.22 | 90.54 | |
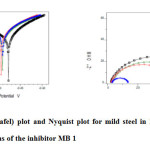 |
Figure5: Polarisation (Tafel) plot and Nyquist plot for mild steel in 1M H2SO4 containing various concentrations of the inhibitor MB 1 Click here to View figure |
Electrochemical Impedance Spectroscopy
Nyquist plots of mild steel in 1 M H2SO4 solution in the absence and presence of different concentrations of the inhibitor MB 1 is shown in Fig. g.The charge transfer resistance (Rct) values were calculated from the difference in the Z1 at low and high frequencies. The inhibition efficiency, (IE %) and the degree of surface coverage (θ) of the Mannich bases can be calculated from the charge- transfer resistance according to Eq. (3) (11):
IE =Rct-Rct*/Rct x 100————————– (3)
Where,Rct* = The charge-transfer resistances for uninhibited solution.
Rct= The charge-transfer resistances for inhibited solution.
Cdl, double layer capacitance obtained from the Nyquist plots and the calculated inhibition efficiency values (IEEIS %) are reported in Table 5. It is apparent from the table that the value of Rct increased with increasing concentration of the inhibitors. The increase in Rct values is attributed to the formation of an insulating protective film at the metal/solution interface. The value of Cdl decreased upon the addition of the inhibitor, suggesting, a decrease in the local dielectric constant and/or an increase in the thickness of the electrical double layer, indicate that the Mannich bases function by the formation of a protective layer at the metal surface(12).
The size of the semicircle increases with inhibitor concentration, indicating that the charge transfer process as the controlling factor of the corrosion of mild steel(13).
Table 5: Impedance parameters for corrosion of mild steel at the selected concentrations of the inhibitor in 1M H2S04
| Name of the compound | Concentration(mM) | Rct (ohm/ cm2) | Cdl (µF)/cm2 | Inhibition Efficiency(%) |
| Blank | 15.08 | 28.2 | ||
| MB 1 | 0.05 | 35.29 | 29.2 | 57.26 |
| 0.3 | 40.14 | 28.6 | 62.43 | |
| 0.5 | 51.31 | 26.3 | 70.61 | |
| MB 2 | 0.05 | 34.65 | 33 | 56.47 |
| 0.3 | 47.74 | 32.3 | 68.41 | |
| 0.5 | 64.36 | 30.8 | 76.56 |
Scanning Electron Microscopy Studies
The surface morphology of mild steel surface was evaluated by scanning electron microscopy (SEM). Figures h, i& j show the scanning electron micrographs of the polished mild steel surface and mild steel immersed in sulphuric acid medium without and with inhibitor MB 1. It is clear that in the absence of the inhibitor, the surface is highly corroded. However in the presence of inhibitor the rate of corrosion is suppressed and the inhibitor forms a protective layer on the surface that prevents the attack of acid on the metal(14).
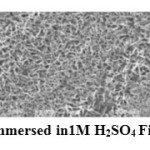 |
Figure 6 Click here to View figure |
Corrosion Inhibition Mechanism
The results obtained from all the studies show that both the Mannich bases have almost same inhibition efficiency, which may be explained as follows. The Mannich bases have almost similar structure except for the substituents on the phenyl ring. The Mannich bases are adsorbed on the mild steel surface through the nitrogen atoms of the benzimidazole ring in a flat orientation. The benzimidazolyl group attached with the t-amino group (dimethylaminomethyl) group gives large steric effect to the disubstituted phenyl ring at position-2 pushing it to a perpendicular plane. Hence the substituents on the phenyl ring have little influence on the inhibition performance.
Conclusion
The synthesized Mannich bases were found to be very good inhibitors for the corrosion of mild steel in 1M H2SO4 and the efficiency increases with increase in concentration of the inhibitor and decreases with temperature. The inhibitors act by adsorption and obey Langmuir Isotherm. Electrochemical studies show that the inhibitors are mixed type.
Reference
- Lagrenee, M.;Mernari, B.; Bouanis, M.; Traisnel, M.; Bentiss, F. Corro. Sci.,2002,44, 573-588
- Quraishi, M. A.;Sardar,R. Corro., 2002, 58,748-755
- Upadhyay, R. K.; Mahur,S. P. E-J. Chem.,2007, 4, 408-414.
- Verma, C. B.; Quraishi, M. A.; Ebenso, E. E. Int.J.ofElectrochem. Sci., 2013,8, 10851-10863
- Praveen, B. M.; Venkatesha, T. V. Int. J. Electrochem. Sci.,4,2009, 267-275
- Dehri, I.;Ozcan, M. Mater. Chem. Phy.,2006, 98, 316-323.
- Popova, S.; Sokolova, A., Raicheva, S.; Christov, M. Corro. Sci.,2003, 45, 33-58
- Bouklah, M.;Benchat, N.; Hammouti, B.;Aouniti, A.;Kertit, S. Mater. Lett.,2007,60,1901-1905.
- Jayaprabha, C.; Sathiyanarayanan, S.;Venkatachari, G. Mater.chem.&phy.,2005, 107,350-355.
- Li,X. H.; Deng, S. D.; Mu,G. N.; Fu, H.; Yang, F. Z. Corros. Sci.,2008, 50, 420-430
- Hamed, E. Mater.Chem. Phys.,2010,121, 70-76.
- El-Haddad,M. N. Int. J. Biolog.Macromol.,2013,55, 142-149.
- Bosch, R.W.; Hubrecht, J.; Bogaerts, W. F.; Syrett,B. C. Corro.,2003, 57, 60-70.
- Hazwan Hussin, M.;Jain Kassim,M. Int. J. Electrochem. Sci., 6,2013,1396-1414

This work is licensed under a Creative Commons Attribution 4.0 International License.









