Effect of Zirconium Dioxide Nanoparticles as a Mordant on Properties of Wool with Thyme: Dyeing, Flammability and Antibacterial
M. Taheri1, L. Maleknia1,*,N. Alizadeh Ghamsari1, A. Almasian2, Gh. Chizari Fard3
1Department of Textile Engineering- Islamic Azad University-South Tehran Branch
2Department of Environmental Research- Institute for Color Science and Technology- Tehran-
3Department of Textile Engineering,Yazd Branch, Islamic Azad University, Yazd, Iran
DOI : http://dx.doi.org/10.13005/ojc/310109
Article Received on :
Article Accepted on :
Article Published : 21 Mar 2015
In this research, the zirconium dioxide nanoparticle was synthesized and the effect of these particles as a mordant on properties of wool fabric in dying process with a natural dye,also flammability and antibacterial properties were studied. The wool fabrics were treated with different concentrations of zirconium dioxide nanoparticles including 1, 3, 6 and 9% o.w.f. and the dyeing process was carried out on the fabrics in the states before, simultaneously and after mordanting with Thyme. The chemical characteristics and the changes induced by zirconium were investigated by Fourier-transform infrared spectra (FTIR).The influence of the amount of nano-ZrO2 and the type of mordanting treatment on dye absorbency were studied by reflectance spectrophotometer (RS). Flammability of samples was investigated by horizontal flammability test (HFT).The antibacterial properties were determined by reduction growth of a Gram-negative bacterium E. coli and a Gram-positive bacterium Staphylococcus aureus. The surfaces of untreated and treated fabrics were evaluated by scanning electron microscopy to observe the morphological changes.
KEYWORDS:Thyme; Nano-ZrO2; Dyeing; Flammability; Antibacterial
Download this article as:| Copy the following to cite this article: Taheri M, Maleknia L, Ghamsari N. A, Almasian A, Fard Gh. C. Effect of Zirconium Dioxide Nanoparticles as a Mordant on Properties of Wool with Thyme: Dyeing, Flammability and Antibacterial. Orient J Chem 2015;31(1). |
| Copy the following to cite this URL: Taheri M, Maleknia L, Ghamsari N. A, Almasian A, Fard Gh. C. Effect of Zirconium Dioxide Nanoparticles as a Mordant on Properties of Wool with Thyme: Dyeing, Flammability and Antibacterial. Orient J Chem 2015;31(1). Available from: http://www.orientjchem.org/?p=7983 |
Introduction
The wool is a natural fiber with the protein structure [1]. We can note the properties of high water absorption (18 percent), reversible capacity due to α-helix structure, and high resistance to fire …. [2].
Natural dyes or colorants derived from flora and fauna are believed to be safe because of their non-toxic, non-carcinogenic and biodegradable nature. The present trend throughout the world is shifting towards the use of ecofriendly and biodegradable commodities. This leads to increase demands for natural dyes [3].Thymus vulgaris (common Thyme, garden Thyme or just Thyme) is a species of flowering plant in the mint family Lamiaceae, native to southern Europe from the western Mediterranean to southern Italy, Growing to 15–30 cm (6–12 in) tall by 40 cm (16 in) wide.It is a bushy, woody-based evergreen subshrub with small, highly aromatic, grey-green leaves and clusters of purple or pink flowers in early summer. The chemical structure of more important compounds, i.e.Thymol and Carvacrol are presented in Fig 1.
 |
Figure1: Chemical structure of the main compounds of Thyme Click here to View figure |
The lack of ability to create a strong linkage of natural dyes with fabrics is one of their disadvantages, herein Thyme is not an exception. Therefore, it is necessary to use a substance as a bridge to improve this defect. Mordant is the substance of helping.in this way two points are important: 1. Mordant ability to create the linkage with the dye and fibers that effects on color fastness, however, the amount of mordant should be optimized; 2. Mordant effects on the shade therefore wide range of mordant can be achieved wide shades.
Recently the most attention in this field is focused on the enzyme treatment and inorganic mordants (AL, Cu, Zn, Ne…) on the wool. However, as far as we know, none of the studies has focused on stabilization of nano zirconia on wool fabric and the influence of these particles on dye absorbency. Attempts were made in the present study to investigate the different physical and chemical properties obtained from coating the nano zirconia on wool fabrics.
Experiments
Materials
The wool fabric with the weight 1688.55 g/m2, width 140 cm was used from Iran Merinos Co.Anionic detergent was provided by SDL Technologies for scouring the wool fabric. Oxalic acid from Merck was supplied for dyeing process. Powder of Iranian Thyme was used for dyeing of wool fabrics. Zirconium oxide chloride (ZrOCl2) and NH4OH were supplied by Merck Chemical Co., Germany.
Preparation of Zirconium Dioxide Nanoparticles
An ammonium aqueous solution was prepared from adding a desired amount of ammonium into the deionized water (pH= 10.5). An aqueous solution of ZrOCl2 (13.05 gr of ZrOCl2 in 100 ml deionized water) was then added into the above solution, and the mixture was vigorously stirred for 30 min (pH= 10.5),then the mixture was centrifuged and washed by successive agitations/centrifugations with deionized water. The obtained wet cake corresponding to prepared nanoparticles was ready to dry at 100˚C for 12 h, and put in furnace at 400˚C for 2 h.
Method
The mordanting treatments were carried out before, simultaneously and after the dyeing process.Zirconium dioxide nanoparticles with the concentrations of %1, %3, % 6 and 9% o.w.f. were used and the liquor ratio was kept at 50:1. The fabrics were maintained for 1h at a temperature of 85◦C. Dye solution was prepared 24 h prior to dyeing process by adding Thyme powder to deionized water(50%o.w.f., liquor ratio 50:1). The dyeing process was started at 40 ◦C and the temperature was increased to 85 ◦C over 20 min and then held at that temperature for 1 h; finally, the fabrics were rinsed with deionized water. The acidic pH was obtained by oxalic acid.
Analytical Methods
Fourier-Transform Infrared Spectroscopy (FTIR)
The chemical compositions of the fabrics were examined by the FTIR spectroscopy [Bomem-MB 100 Series (Hartmann and Broun)rsqb].
Microscopic Characterization
The surface of the fibers was investigated using a Scanning Electron Microscope (SEM XL30, Philips). The surface of samples was first coated with a thin layer of gold (w10 nm) by Physical Vapor Deposition method (PVD) using a sputter coater (SCDOOS, BAL-TEC). The presence of zirconia on fiber surface was also determined by energy dispersive X-ray microanalysis (EDX) attached to the SEM.
The Vertical Drop Test
The vertical drop test was carried out on fabrics with standard BS 4554. A drop of distilled water was placed onto the fabric sample and the time by which the liquid required to sink completely into the fabric was recorded. The shorter the time, the more wet table was the fabric.
Color Coordinates
CIELAB color coordinates (L*, a*, b*, C* and h) were calculated for control and treatment samples at 10° observer and illuminant D65.
Determination of Flammability
Flammability of samples was evaluated in accordance with ASTM D 635. Five samples from each fabric were cut with dimensions of 10×2 cm. Specimens were placed in the flammability chamber in the horizontal position (Fig 2). After testing, the time and extent of burning area were measured and the rate of burning (V) was calculated in millimeters per second (mm/s) for each specimen by the following formula:
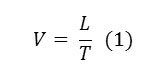
where L is the burned length in millimeters (mm) and T is the time in seconds (s) [4].
 |
Figure2: Flame chamber used in this study with horizontal position for samples
|
Evaluation of Antibacterial Efficiency
The antibacterial activity of the treated wool fabric was tested against two bacteria, Staphylococcus aureus (S. aureus), American Type Culture Collection No.6538, as a gram positive bacterium and Escherichia coli, American Type Culture Collection No.11303, as a gram negative bacterium. To this end, some colonies of each bacterium were suspended in a physiologic saline solution (NaCl 0.9% in distilled water at pH 6.5) with concentration of 0.5 Mc Farland. The vials of bacterial suspensions were then incubated with the agitation at 37ºC ± 2ºC, 220 rpm for 2 hours. A homogenous suspension of bacteria was prepared and serial dilution was then done in 5 Steps (dilution of 1:100000). A concentration of about 1.5-2Í10³ CFU/ml was applied for the antibacterial testing. The bacteriological culture tubes (ie, 125Í17mm glass tubes), containing one piece of the treated wool fabric (10mmÍ10mm) were sterilized by an autoclave device in moisturized heat (121º C, 15 Ib) for 15-20 minutes. An aliquot of 1cc bacterial suspension and 2cc of TSB (Tryptic Soy Broth) was then added to every tube and 3 ml was detected in each tube. To ensure that any decrease in bacterial count was due to the exposure to the wool fabrics, one control of the saline solution with TSB, and one control of aliquot with the untreated fabrics including the tubes containing the treated wool fabrics with the bacterial suspensions and the control tubes were incubated at 37ºC for 24h. The samples of 10 µl from each tube were then taken and counted according to the pour plate method. Thus, samples were mixed with melted agar (that decreases the temperature down to 45ºC) and poured. The plates incubated at 37ºC for 24h and the colonies of each plate were counted by a colony counted device to determine the bacteria reduction of the suspensions. The results of the numbers before and after the treatment with the wool fabrics were used to determine the bactericidal effect and the percentage of reduction of bacteria was calculated using the following equation:
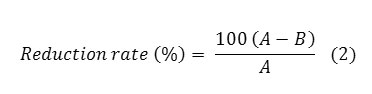
Where R is the bacterial reduction ratio, A is the number of bacterial colonies from the untreated fabrics and B is the number of the bacterial colonies from the treated fabrics.
Evaluation of Bending Rigidity
In this test, a rectangular strip of fabric is supported on a horizontal platform in a direction perpendicular to one edge of the platform (BS 3356). The strip is extended in the direction of its length so that an increasing part overhangs and bends don under its own mass. When the tip of the specimen has reached a plane passing through the edge of the platform and inclined at an angle of 41.5° below the horizontal, the overhanging length is equal to twice the bending length of the specimen. The flexural rigidity (G) is obtained from the bending length (C) and the mass per unit area of the fabric (M).
G = M × C3 × 0.1 (3)
Results and Discussion
Characterization of Nanoparticles
FTIR Analysis
The infrared spectrum of nanoparticles is shown in Fig 3.Bands observed at 3211 cm-1 and 1564 cm-1 are assigned to the stretching and bending vibrations of the O-H bond due to absorbed water molecules, respectively. The band at 1337 cm-1is attributed to the absorption of non-bridging OH groups [5]. The features particularly at 752 cm–1 and 466 cm–1, due to Zr–O2–Zr asymmetric and Zr–O stretching modes respectively, confirm the formation of ZrO2 phases [6].
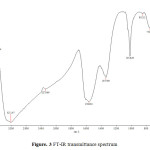 |
Figure3: FT-IR transmittance spectrum Click here to View figure |
SEM Analysis
SEM is one of the best methods to study the nanoparticles morphology. Fig.4 shows the SEM photographs of the nanoparticles having different particle sizes. The flaky layers of zirconium particles have a thin structure of approximately 72 nm, showing a high aspect ratio. SEM results indicated that ZrO2 possess a nearly coarse spherical morphology with a heterogeneous distribution
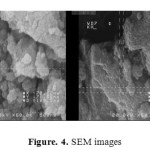 |
Figure4: SEM imagesClick here to View figure |
Study of Fabrics Dyeing
Structural Information by FTIR Spectra
The infrared spectra of untreated wool as well as the mordanted sample with zirconium dioxide nanoparticles are shown in Figures 5. The N-H stretching and bending vibrations in wool are usually appeared at 3100-3500 cm-1 and 1550-1640 cm-1 respectively, depending on the type of amide (primary and secondary), chemical environment (solid and liquid) and intra- or inter-molecular hydrogen bonds. The C=O stretching vibration band appears in the normal region between 1630 and 1670 cm-1 which usually overlaps with N-H bending [7,8].
In Fig 5a N-H stretching of a secondary amide is appeared at 3251 cm-1as expected for wool. The band at 1634 cm-1 is also characteristic of N-H bending vibration which overlaps with C=O vibration bond. The weak C-H stretching, CH2 out of plane bending, C-O-C asymmetric stretching and S-O-S (cystine monoxide) vibrations in wool were appeared at 2919, 835, 1232 and 1072 cm-1, respectively. Other weak bands appeared at 3759 and 3682 cm-1 are attributable to O-H stretching vibrations in free primary and secondary alcohols [8].
As can be seen in Fig 5b, dyed wool structure was the same as untreated wool. The intensity of the band at 3853cm-1was increased and a new band at 793 cm-1was appeared. These changes are due to interaction between OH of the dye and amide bond of the wool, also it can be stated this increasing is due to absorbed dye molecules and increment of OH bonds on the surface of wool.
As can be seen in Fig 5c, innano zirconium dioxide / Thyme/ wool sample, the band at 1391 cm-1 was decreased as compared to untreated wool. This could be attributed to change of CH bending vibrations due to incorporation of ZrO2 nanoparticles on the surface of wool fabric, decrement in the intensity of band at 3516 cm-1 can be expressed the interaction between OH of Thyme and wool on one hand and mordant particles on the other hand causing to decrease free OH groups in wool.
 |
Figure5: mentioned in the figure caption list should be cited and provided sequentially.igure 5: FTIR spectra a) untreated wool, b) dyed wool with Thyme, c) 6% nanozirconium dioxide / Thyme / wool Click here to View figure |
Microscopic Characterization
The SEM images of the wool fiber with Thyme and wool/Thyme/nano-zirconia samples are shown in Fig 6a-d. It can be observed that dyed fiber with Thyme has overlapping scales with no deposition of the nano-particles.Images of wool/Thyme/nano-zirconia fibers showed the formation of aggregated nano-zirconia minerals on the surface of wool [9].
As can be seen the presence of nano zirconia is more obvious in dyed wool fibers with nano zirconia in after – mordant method, probably in two other methods some particles are removed during the processes.
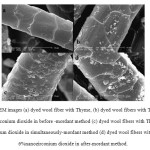 |
Figure6: SEM images (a) dyed wool fiber with Thyme, (b) dyed wool fibers with Thyme and 6%nanozirconium dioxide in before -mordant method (c) dyed wool fibers with Thyme and 6%nanozirconium dioxide in simultaneously-mordant method (d) dyed wool fibers with Thyme and 6%nanozirconium dioxide in after-mordant method. Click here to View figure |
Figs. 7a-d and Table 1 showed the presence of chemical elements existing on the surface of untreated fiber and the dyed wool fibers with Thyme and nano zirconia. In these patterns, Au peaks clearly showed that gold is successfully coated on surfaces of all fibers; and there is no Zr present on untreated wool. TheEDX analysis of treated wool with nano- ZrO2 illustrates the presence of Zr on surface of wool in all mordanting method, increasing from before-mordant method to after treatment method. It is obvious the after-mordant method caused to aggregate more ZrO2 particles on the surface.
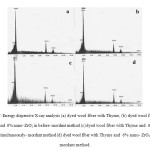 |
Figure7: Energy dispersive X-ray analysis (a) dyed wool fiber with Thyme, (b) dyed wool fiber with Thyme and 6% nano- ZrO2 in before- mordant method (c) dyed wool fiber with Thyme and 6% nano- ZrO2 in simultaneously- mordant method (d) dyed wool fiber with Thyme and 6% nano- ZrO2 in after- mordant method. Click here to View figure |
Several factors effect on ability of nano-particles to aggregate on the surface of textiles including size, mobility, end-group functionalities, relative composition and molecular architecture. It seems that the interactions between nano-particles and fibers are strong enough to enable deposition of nano-zirconia particles on the wool surface as a result of their high surface area. Other authors have confirmed this phenomenon in cases where the nano-particles were incorporated into the multilayer organic coatings [10,11, 12] .
Table 1: Energy dispersive X-ray analysis (a) dyed wool fiber with Thyme, (b) dyed wool fiber with Thyme and 6% nano- ZrO2 in before- mordant method (c) dyed wool fiber with Thyme and 6% nano- ZrO2 in simultaneously- mordant method (d) dyed wool fiber with Thyme and 6% nano- ZrO2 in after- mordant method.
|
Au(%) |
Zr(%) |
method |
|
|
91.94 |
8.06 |
Before- mordant |
|
|
91.30 |
8.70 |
Simultaneously- mordant |
|
|
90.10 |
9.90 |
after-mordant |
|
Water Drop Absorption Time
Fig8showed the measured times for water dropletto completely absorb on dyedwool fabrics with Thyme and different concentrations of nano-zirconia. The absorption and desorption of water from wool is related with the concentration and type of functional groups in the protein chains [13,14,15, 16]. On the other hand, the hydrophilicity or hydrophobicity of nano-zirconia depends on its specific surface area, average particle size and type of packing of primary particles in aggregates[19,20, 22]
In this study, the hydrophobic properties of wool achieved through the creation of linkage between protein chains, Thyme and nano-zirconia. The results indicate that absorption time increases with increment of the concentration of nano-zirconia. After-mordant method illustrates more absorption time than two other methods, it seems that using nano-zirconia as after-treatment caused to more hydrophobicity.
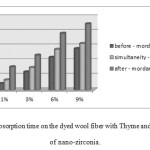 |
Figure8: Water droplet absorption time on the dyed wool fiber with Thyme and different concentrations of nano-zirconia. Click here to View figure |
Colorimetric Measurements
The color values were evaluated in CIELAB color space, the three axes being L*, a*, and b*. The L* is the color coordinate which represents the lightness of samples and can be measured independently of the color hue. Any decrease in the lightness of samples could be concluded as the lower reflectance of textiles. The a* stands for the horizontal red–green color axis. The b* represents the vertical yellow–blue axis. The C* shows the brightness or dullness of the samples. Any increase in the C* of samples could be concluded as greater brightness of the sample. The hue angle (h°) stands for hue, which is the actual color recognized by the human eye and identified as orange, yellow, beige, brown, pink or any of the other colors visible to humans. It is expressed in degrees, with 0° being a location on the +a* axis, continuing to 90° for the +b* axis, 180° for -a*, 270° for -b*, and back to 360° = 0°.
The L*, a*, b*, C* and h values of nano – ZrO2 / Thyme / woolsamples and unmordanted one are given in Table 2.As can be seen,the lightness (L*) values decreased in before and simultaneously-mordant methods and in after-mordant method was not significant. Decreasing in L* values could be the results of more dyes penetration into the fabrics which allows the results of exhaustion values. With increasing the concentration of nanoZrO2, the amount of absorbed dye increased until 6%. It can be stated that due to increment of hydrophobicity effect in higher concentration of nanoZrO2 the exhaustion value decreased. There existed a change in a*, b*, C* and h for nano – ZrO2 / Thyme / wool fabrics, and an increment of yellow is seen in after-mordant method.
Table 2: Color coordinates of unmordanted and nano – ZrO2/ Thyme / wool
|
Before-mordant |
Simultaneously-mordant |
after-mordant |
|||||||||||
|
|
0% |
1% |
3% |
6% |
9% |
1% |
3% |
6% |
9% |
1% |
3% |
6% |
9% |
|
L* |
58.5 |
57.0 |
56.8 |
53.8 |
62.4 |
57.8 |
57.4 |
54.9 |
61.7 |
59.4 |
60.0 |
59.7 |
59.6 |
|
a* |
22.6 |
26.6 |
22.3 |
21.6 |
21.3 |
20.5 |
19.7 |
20.4 |
20.5 |
22.6 |
20.9 |
19.8 |
19.7 |
|
b* |
29.8 |
33.4 |
25.4 |
17.2 |
15.8 |
27.7 |
26.9 |
25.8 |
19.9 |
31.7 |
33.5 |
32.8 |
32.5 |
|
C |
37.4 |
40.3 |
33.8 |
27.6 |
26.5 |
34.5 |
33.3 |
32.9 |
28.5 |
39.0 |
39.5 |
38.3 |
38.0 |
|
H |
52.8 |
55.9 |
48.7 |
38.6 |
3605 |
53.5 |
53.8 |
51.7 |
44.2 |
54.5 |
58.1 |
58.9 |
58.8 |
The results obtained from color coordinates of samples indicated that mordant concentration and mordanting method have a direct influence on shade.
Determination of Flammability
Table 3 shows the summary of the length, time and rate of burning for untreated and treated wool textiles. The images for untreated and treated wool fabrics after the flammability test are also given in Figs 9. The longer burning length and higher burning rate indicated the greater the flammability. The untreated wool fiber ignites easily after exposing to flame. However from Table 3, it is clear that in the treated samples the burning lengths were decreased and the burning rate was significantly lower than untreated one.
It can be seen that the time needed for burning of the same length for treatment samples were longer compared with untreated wool.
Table 3: Burning values for different methods with 6% nano zirconia
|
sample |
Burned length (mm) |
Ignition time (s) |
Burning rate (mm/s) |
|
no mordant |
80 |
2.71 |
29.52 |
|
Before-mordant |
26 |
5.1 |
5.09 |
|
Simultaneously-mordant |
23 |
7.8 |
2.95 |
|
after-mordant |
18 |
9.4 |
1.91 |
The wool is formed by fibrils, with helical coiled molecules, embedded in the amorphous matrix. The fibrils and the matrix consist of helical polypeptides chains interconnected by hydrogen, electrostatic and covalent linkages. The thermal properties and flammability of this natural polymer are influenced by its molecular structure, density, crystallization level, crystal orientation angle and mobility of molecular chains in amorphous and crystalline regions. Since mordanted wool with nano zirconia and dyed with Thyme cause some changes in chemical, physical and morphological characteristics of fiber thus changes in its flammability characteristics could also be expected.
 |
Figure9: Images of wool fabrics after the flammability test (A) untreated wool, (B) Before-mordant, (C) simultaneously-mordant (D) after-mordant Click here to View figure |
Antibacterial Properties of Wool Samples
The antibacterial effect of natural dye depends onphenelic, polyphenol,phelaonol, tanon, komarin and poly peptid groups in antibacterial compounds.The antibacterial effect of Thyme depends to very antibacterial compounds in plant and metabolic toxins. Fig 10 and Table 4 depicts the colonies of S. aureus and E. coli corresponding untreated wool and the treated samples.The results showed that Thyme and nano zirconia have a positive effect on antibacterial properties of the treated textile samples.The reduction rates for both Gram-positive and Gram-negative bacteria were acceptable.
Table 4: Antibacterial test result against E. coli and Staphylococcus aureus A) untreated wool B) Before-mordant, C) simultaneously- mordant, D) After- mordant
|
sample |
Staphylococcus aureus |
E.Coli |
||
|
Number of colony |
Reduction of bacteria (%) |
Number of colony |
Reduction of bacteria (%) |
|
|
no mordant |
∞ |
0% |
∞ |
0% |
|
Before-mordant |
360 |
43.75% |
400 |
32.10% |
|
Simultaneously-mordant |
7 |
98.90% |
21 |
96.44% |
|
after-mordant |
3 |
99.53% |
17 |
97.11% |
The results showed that Thyme dye has an adhesive ability to microorganisms. They can be used in removal of a diverse range of biological contaminants including bacteria, viruses and natural organic matters. This is due to Thyme antibacterial compounds which impinge the bacterial cell surface, disrupting the intracellular metabolic pathways. Subsequently, the internal contents are released due to the cell rupture caused by the oxidative stress.
 |
Figure10: Antibacterial test result against E. coli (right) and Staphylococcus aureus (left) A) untreated wool B) Before-mordant, C) simultaneously- mordant, D) After- mordant Click here to View figure |
Determination of Bending Rigidity
The bending rigidity of non-treated and nano zirconia treated wool samples are illustrated in Fig 11. As can be seen, a 6%concentration of nano zirconia decreased the bending rigidity of fabric and decreased the bending length, hence improved the handle of wool fabrics.
Drape is one of the important factors influencing the functionality and handle of textiles. It is defined as a phenomenon of fabric-fold formation, which arises when a fabric hangs down without the influence of external forces. The important influencing factors on the fabric’s drapeability are its structure, yarn type, fiber content, shear, form ability, fabric weight as well as its finishing treatments. The greater the fabric weight, the better the drapeability.
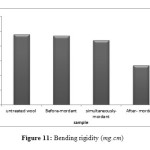 |
Figure11: Bending rigidity (mg.cm) Click here to View figure |
Conclusion
Nano zirconia and Thyme was used for dyeing of wool through before, simultaneously and after-mordantingmethods. The FTIR spectra showed that nano zirconia increased the interfacial interactions and bonding with the amine or hydroxyl end groups of wool chains by interactions and conformation changes. The results obtained from the LOI test demonstrated a decrement of flammability of mordanted samples. This can be as a result of the heat insulation effect of nano zirconia particles embedded in fibers. The hydrophobic properties increased in mordanted samples that can be attributed to the presence of nano zirconia. It was also indicated that wool fabrics have a good antibacterial for both Gram-positive and Gram-negative bacteria.
References
- Bearpark, F.;A practical introduction to the dyeing and finishing of wool fabrics. William Marriott And J. Park, 1996, 43-50.
- Simpson, W.S.; Crawshaw G.H.; Wool: Science and Technology. CRC Press,2002,pp60-70.
- Farizadeh K.,Yazdanshenas M.E.,Montazer M.,Malek R.M.A. , Rashidi A., Kinetic Studies of Adsorption of Madder on Wool Using Various Models. Text Res J, 2010,80:847–855.
- Parvinzadeh M., Eslami S., Optical and electromagnetic characteristics of clay–iron oxide nanocomposites. Res. Chem. Intermed,2011, 73:771-784.
- Jayakumar S., Ananthapadmanabhan P.V. , Perumal K., Thiyagarajan T.K., Mishra S.C., Su e L.T., Tok A.I.Y. ,Guo J,Characterization of nano-crystalline ZrO2 synthesized via reactive plasma processing. Materials Science and Engineering: B,2011, 176: 894-899.
- H ranjansahu,Grangarao, Characterization of combustion synthesized zirconia powder by UV-vis, IR and other techniques. Bull. Mater.Sci,2000, 23: 349-354.
- Parvinzadeh M., Assefipour R., Kiumarsi A.,Biohydrolysis of nylon 6,6 fiber with different proteolytic enzymes. Polym.Degrad. Stabil,2009,94: 1197-1205.
- Parvinzadeh M., Effect of proteolytic enzyme on dyeing of wool with madder.Enzym.Microb.Technol,2007, 40: 1719-1722.
- Sipaut C.S., Ahmad N., Adnan R., Rahman I.A.B., Bakar M.A., Ismail J., Chee C.K., Properties and Morphology of Bulk Epoxy Composites Filled with Modified Fumed Silica-Epoxy Nanocomposites. J. Appl. Polym. Sci, 2007, 7 : 27–34.
- Geoghegan M., Krausch G., Wetting at polymer surfaces and interfaces. Prog.Polym.Sci, 2003 ,28: 261–302.
- Parvinzadeh M., Ultrasonic Assisted Finishing of Cotton with Nonionic Softener.TensideSurfactDet,2009 ,46: 335-339.
- Parvinzadeh M., Memari N., Shaver M., Katozian B., Ahmadi S., Ziadi I., Influence of Ultrasonic Waves on the Processing of Cotton with Cationic Softener. J. Surfact. Deterg.,2010, 13: 135-141.
- Bingshe X., Mei N., Liqiao W., Wensheng H., Xuguang L., The structural analysis of biomacromolecule wool fiber with Ag-loading SiO2 nano-antibacterial agent by UV radiation. PhotochemPhotobiol A: Chemistry ,2007 , 188 : 98–105.
- Montazer M., Pakdel E.,Reducing Photoyellowing of Wool Using Nano TiO2.PhotochPhotobiol,2010 , 86: 255–260.
- Dastjerdi R., Montazer M., Shahsavan S., A novel technique for producing durable multifunctional textiles using nanocomposite coating. Colloids Surfaces B: Biointerfaces,2010 , 81: 32- 41.
- Montazer M., Pakdel E., Moghadam M. B., The role of nano colloid of TiO2 and butane tetra carboxylic acid on the alkali solubility and hydrophilicity of proteinous fibers. Colloids Surfaces A: Physicochemical and Engineering Aspects,2011, 375: 1-11.
- Montazer M., Behzadnia A., Pakdel E., Rahimi M. K., Moghadam M. B.,Photo induced silver on nano titanium dioxide as an enhanced antimicrobial agent for wool. Journal of Photochem and Photobiol B: Biology,2011, 103: 207-214.
- Montazer M., Behzadnia A., Pakdel E.,Novel feature of nano-titanium dioxide on textiles: Antifelting and antibacterial wool. Applied PolymSci,2011, 121: 3407–3413.
- Srivastava A., Dongare M.K.,Low-temperature preparation of tetragonal zirconia. MaterLett ,1987,5: 111-115.
- Chen S. Y., Jang L. Y., Cheng S., Synthesis of Thermally Stable Zirconia-Based Mesoporous Materials via a Facile Post-treatment. J. Phys. Chem. B,2006 , 110 :11761-11771.
- Hsu Y.S, Wang Y.L., Ko A.N.,Effect of Sulfation of Zirconia on Catalytic Performance in the Dehydration of Aliphatic Alcohols.J. Chin. Chem. Soc., 2009 , 56: 314-322

This work is licensed under a Creative Commons Attribution 4.0 International License.









