Effect of FeSO4.7H2O and SnCl2.2H2O added as Chromium (VI) Reducers in Ordinary Portland Cement
Devesh K. Sharma* and Rekha Sharma
School of Physical Science, Lovely Professional University, Phagwara (Panjab), India
DOI : http://dx.doi.org/10.13005/ojc/310164
Article Received on :
Article Accepted on :
Article Published : 25 Feb 2015
The paper compared the effect of additives such as FeSO4.2H2O and SnCl2.2H2O on reduction of water soluble Cr(VI), in ordinary Portland cement (43 grades). The determination of water soluble Cr(VI) from cement samples was precisely conducted by DPC-Spectrophotometer technique. The compressive strength testing of the cement mortar samples was carried out. In addition, hydration properties of cement paste (with and without additives) are reported in this paper. Hydration products were also studied in detail by TGA, SEM and XRD. Tin chloride was found to be the most efficient additive for reducing Cr(VI) in cement. XRD confirmed two phases such as monocarbonate and Friedel’s salt (at 2θ = 11.5) were formed during cement hydration in the presence of tin chloride but not confirmed the presence of calcium hydroxo-stannate. These studies showed significant retardation in early days hydration.
KEYWORDS:Cr (VI); Spectrophotometer; TGA; SEM; XRD; compressive strength
Download this article as:| Copy the following to cite this article: Sharma D. K, Sharma R. Effect of FeSO4.7H2O and SnCl2.2H2O added as Chromium (VI) Reducers in Ordinary Portland Cement. Orient J Chem 2015;31(1). |
| Copy the following to cite this URL: Sharma D. K, Sharma R. Effect of FeSO4.7H2O and SnCl2.2H2O added as Chromium (VI) Reducers in Ordinary Portland Cement. Orient J Chem 2015;31(1). Available from: http://www.orientjchem.org/?p=7450 |
Introduction
Cement is a fine, soft, powder being used worldwide as building material for their strong binding properties when mixed with water, but this binding material contains water soluble hexavalent chromium, which generally causes skin irritation and allergic eczema to workers who come into contact with cement or cement materials (1). According to COSHH (control of substances hazardous to health) regulation, the allowed level as Cr (VI) in cement is less than 2.0 ppm (2). For the reduction of toxic Cr(VI) into nontoxic form Cr(III), a number of reducing agents like ferrous sulphate heptahydrate (HH) and monohydrate (MH), sulphur compounds (metabisulphite S2O52-, sulphite SO32-, and thiosulphate S2O32-, Na2S2O4, NaHSO3, SnSO4, SnCl2·2H2O, antimony (III) compounds, amine based compounds (hydrazine, hydroxylamine), ammonium ferrous sulphate (formula), MnSO4, FeS, solid lignin (SL), EDTA, NaBH4 etc have been studied (3-9). Among these reducing agents, salts of iron and tin are widely used (10).
High dosage of reducing additive required the addition during grinding and packaging to get the desired result (to use up to six months) by maintaining the concentration that may cause overdosing and retard the hydration process as well as cement strength (10).
Additions of tin salt were expected to lead to the formation of additional hydration product. Ettringite is particularly noticeable in the sample admixture with iron sulphate. The reaction of tin chloride to form of calcium hydroxo stannate, CaSn(OH)6, and Friedel’s salt (tetracalcium aluminate dichloride-10-hydrate), Ca3Al2O6.CaCl2.10H2O (11), is well documented at 5% (w/w), but not at 1% concentration of tin chloride.
As far as we are aware, the comparable study in the presence of tin chloride and iron sulphate salts has not been reported. Thus, keeping these problems in mind the current research work has been aimed to study the effect of reducing agents (FeSO4.7H2O, SnCl2.2H2O) added at the time of utilization, on their reducing efficiency, compressive strength of cement mortar and hydration process of cement paste to understand the interaction of additives.
Material and Methods
Materials
The Ordinary Portland Cement (OPC, 43-Grade of ACC brand) used for this work was obtained from the project site of National Highways Authority of India in the state of Uttar Pradesh (at district Etawah). Indian standard Sand (TAMIN, Ennore) as per IS 650 was used in this experiment, there are three grades of this sand (from 1.0 to 0.09) Physical analysis and chemical composition of OPC has been carried out in Oriental Structural Engineers Pvt. Ltd and data obtained are presented in Tables 1 and 2 respectively.
 |
Table 1 Click here to View table |
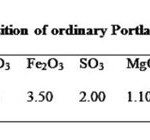 |
Table 2 Click here to View table |
Reagent and Solution
All the reagents and chemicals such as diphenylcarbazide (DPC), K2Cr2O7, FeSO4.7H2O and SnCl2.2H2O, ethanol, phthalic anhydride, H2SO4 used in the study were of analytical/G.R. grade, which were purchased from Merck (Mumbai, India) except DPC which was purchased from Himedia chemicals (Mumbai). The 0.25% diphenylcarbazide (DPC) solution was prepared as per the procedure followed by 1.0 gram of diphenylcarbazide (DPC) was dissolved in 75 mL ethanol. To this was added 5 gram phthalic anhydride and 6 drops of con H2SO4 and made up to 100 mL with ethanol. Standard stock solution of 100 ppm Cr (VI) was prepared by dissolving 0.283 gram of K2Cr2O7 in one liter distilled water having an electrical conductivity 2.3 mS (HANAA H 18733) and its pH at 29.3ºC was 7.66. This stock solution was used for the preparation of working solutions. After that, 2.0 ml of DPC solution (0.25%) and 2.0 ml of 6N sulfuric acid were added. DPC causes reduction of Cr (VI) to Cr (III) by itself undergoing oxidation. Thus DPCA and Cr (III) formed a magenta colored complex. Concentration of this complex was measured by the spectrophotometer with a maximum absorption at 540 nm (12, 13). From the absorbance corresponding concentration in ppm was found from the calibration curve.
Determination of Water Soluble Cr (VI)
For the extraction of the water soluble Cr (VI), 2.0 g sample was taken in a 250 ml beaker and 100 ml distilled water was added to it. The contents were thoroughly mixed with a glass rod. The beaker was covered with a watch glass. The contents were mixed intermittently after every day hour. This process was continued for five days. The contents were filtered with two times washing through Whatman 42 in a 100 ml volumetric Flask (12). After extraction samples were analyzed by standard DPC method (13, 14). Measurement of soluble Cr (VI) in ordinary Portland cement before and after adding the reducing additives, Additives (such as FeSO4.7H2O and SnCl2.2H2O) were mixed in cement for reduction of water soluble Cr (VI).
Determination of Compressive Strength
200 gram Portland cement with additives at different quantity (0.1, 0.5 and 1.0%) was mixed with standard sand in 1:3 ratios and added water (w/c ratio was 0.40). The mortars were placed in steel moulds to form cubes, 70.6 mm3 in dimension. These cubes were demoulded after 1 day and stored in water at 27°C at a relative humidity of 100%. The cubes were taken out of the water prior to testing. The compressive strength was determined at 3, 7 and 28 days as per IS: 4031 part 6, 1988. The details of cement samples are presented in Table 3.0.
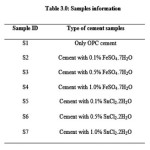 |
Table 3 Click here to View table |
Preparation of Hydrated Samples
To analyse the interaction of additives with Portland cement therefore going further for microscopic studies, in this case, 1.0 % of the both additives were blended in cement with w/c ratio was 0.40. All the material was mixed thoroughly and formed cement paste which was sealed in polythene bags (cement samples as S1, S4 and S7). Hydration of OPC in polythene bags started and making until all the water was consumed (at 28 days) and curing process was not done. After that these samples were analyzed through Thermo-gravimetric analysis (TGA), Scanning Electron Microscopy (SEM), X-ray Diffraction method (XRD).
Thermo-Gravimetric Analysis (TGA)
Thermo gravimetric Analysis (TG) determines the weight changes of a sample. TG studies of hydrated cement in the presence and absence of additives were recorded with Perkin Elmer, Diamond at a heating rate of 10°C/min
Scanning Electron Microscopy (SEM)
Scanning electron microscopy (SEM) images of hydrated Portland cement in presence and absence of additives were recorded with the help of JEOL Model JSM – 6390 LV scanning electron microscope.
X-ray Diffraction Method (XRD)
X-Ray Powder Diffractometry of hydrated cement samples with and without additives was recorded. For this, AXS D8 advane diffractometer equipped with a Si(Li)PSD detector including X-ray source of Cu Kα radiation (λ = 1.5418 Å) was used. The scan step size was 0.024°, the collection time 65.6 s, and in the range 2Θ CuKα from 3° to 80°. The X-ray tube voltage and current were fixed at 40 kV and 35 mA respectively.
Result and Discussion
Reduction of Water Soluble Cr (VI) in Hydrated Cement Samples
The comparative analysis was carried out to know the effect of chromium hexavalent reducing additives such as ferrous sulphate (FeSO4 .7H2O) and stannous chloride SnCl2.2H2O on the reduction of Cr (VI) by measurement of Cr(VI) before and after adding the reducing agent in cement samples. The results obtained after the action of reducing agents and the reduction of hexavalent chromium are shown in Table 4. With 1.0 % dosages of SnCl2.2H2O was better to reduce 25 ppm Cr(VI) completely, present in hydrated cement therefore use of SnCl2.2H2O, described higher reduction efficiency over iron salts. In present research, the amount of additives used slightly higher as per previous work (15), the cause for this may be the use of additives in their crystalline form. Therefore addition rate is usually varied with content of water soluble Cr(VI) as well as the form of additives (10).
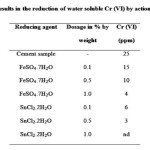 |
Table 4 Click here to View table |
Compressive Strength Studies
The effect of chromium-reducing agents on the compressive strength of 1:3 cement-sand mortars has been determined at 3, 7 and 28 days. The results are given in Table 5. Incorporation of additives with different dosages were retarded the initial hydration process therefore lower in compressive strength of 3 days with additives but no problems with 28 days strength of cement mortar except cement samples contain 1.0% ferrous sulphate additive (S4 sample) as per IS: 8112-1989. In the case of FeSO4, the cement mortar with 1% admixture are strongly retarded therefore samples are lower in compressive strength test. However, at 1% SnCl2 in the cement mortar, the compressive strength increases after 7 days curing as well as at later age is observed. Large size of sulphate ion (iron sulphate) may retard the hydration process therefore lower in cement strength whenever small size of chloride ion (tin chloride) which enhanced the hydration process as well as strength of cement mortar, including soluble salts SiOCl2 might have been produced and crystallization of these salts might have been taken place in the pores. An another cause for retardation in initial hydration process is larger size of metal ions (tin and iron) involved in crystallization of ettringite leading to increase in voids (16).
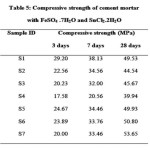 |
Table 5 Click here to View table |
Effect of Additives on Hydrated Cement
Since the cement sample with 1% (w/w) dosage level of SnCl2.2H2O shows good efficacy with respect to Cr (VI) reduction and good compressive strength of cement mortar at 28 days (S7), further investigations using TGA, SEM and XRD techniques were carried out in order to understand phase alterations during hydration of cement with and without additives.
Thermo-Gravimetric (TGA) Studies
The Calcium hydroxide content of the cement mortars was determined by the thermo gravimetric analysis (TGA). In all hydrated samples, the peak corresponding to Ca(OH)2 was observed in between 420 to 431ºC (13) as shown in Figure 1. The generation of Ca(OH)2 in hydrated cement with 1% additives is shown in Table 6. The percentage of Ca(OH)2 in hydrated cement with FeSO4 .7H2O and SnCl2.2H2O was lower than in hydrated Portland cement (S1). The minor shift in the peak temperatures value was observed which may arise due to change in cement microstructure (17). In sample S4, the hydration reaction occurred to a slight degree therefore a very small amount of Ca(OH)2 was obtained. However, Sample S7 showed good agreement with sample S1. It can be seen that a small broad peak between 50°C and 200°C, which illustrates the minor development of calcium silicate hydrate phases (C-S-H) in these samples but very less in S4 samples (18,19).
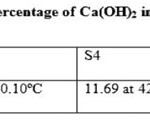 |
Table 6 Click here to View table |
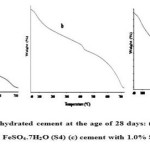 |
Figure 1 Click here to View figure |
Microscopic Studies
Qualitative information on (Calcium silicate hydrate) CSH gels was obtained from SEM morphology of hydrated samples with and without additives at 28 days, which are presented in Figure 2 (a, b and c) respectively. Amorphous CSH gel (light color), which is the main hydration product, was observed in hydrated cement samples. Dicalcium silicate hydrate phases are in spherically small size but tricalcium silicate hydrate phases are in large rectangular size (17). Presented research exhibited the hydrated calcium silicate phases as shown in morphology of hydrated cement samples, this indicating that the formation of hydration products (19).
 |
Figure 2 Click here to View figure |
Hydration product decreased in hydrating cement having 1% FeSO4.7H2O (S4). This delay in hydration due to curing of cement paste has not been done as well as deficiency of proper silica (sand) for reaction to make calcium silicate hydrate gel, it provides strengthen to cement materials. Thermal analysis (TGA) are also supported the SEM morphology for the formation of high amount of calcium hydroxide in Portland cement sample (S1), but in cement sample with 1% tin salt additive (S7) showed that the formation of rigid surface as well as crystal of hydrated tricalcium silicate phase (20). More rigid large structure of sample S7 in the SEM morphology (Figure 2c) and evidence for a higher degree of silicate polymerization, these observations well coincide with the compressive strength results (given in Table 5) and morphological analysis results (Figure 2). During hydration of Portland cement without additives liberated high amount of free lime in the cement materials by which leads to the expansion of small cracks [17].
X-ray Diffraction (XRD) Studies
The XRD patterns of OPC hydrated with and without additives are presented in Figure 3 (a, b and c) and found their relative intensity of the main peaks of Calcium hydroxide (CH) at (2θ = 18.2º, 34.2° and 47.32), Tricalcium silicate (C3S) at (2θ = 29.6°), dicalcium-silicate (C2S) at (2θ = 32.32) and ettringite phase at (2θ = 9.35). If we look in XRD spectra, the total intensity of CH formation in S1, S4 and S7 samples are 3845, 2918 and 3093 counts. This results confirmed that the intensity of Portland cement without additives showed more in hydration (due to high intensity of CH) as compare to other two samples (S4 and S7) but as per SEM, they supported to S7 samples for completion of hydration in 28 days except some crystals transformation into solid rigid like had not been done in surface. An extra peak was also found in S7 sample (at 2θ = 11.5). This peak indicated about both monocarbonate as well as Friedal’s salt (Ca3Al2O6.CaCl2.10H2O) formation which showing that clearly about hydration process.
Monocarbonate phase decreases the porosity of cement materials and helping in enhancing the strength of cement materials after 28 days (21) but in present research work, this phase has been found at 28 days therefore this indicated about good strength of cement mortar sample (S7) at 28 days. Monocarbonate phase is destabilized to monosulphate and calcite at above 47ºC which leads to a higher coarse porosity and reduces the compressive strength of cement samples (22). Samples S1 and S4 may be released more energy during hydration therefore monocarbonate phase was not found in their XRD spectra. This would be the reason for less compressive strength of S1 and S4. If compared with S7 samples at 28 days.
Cement mortar samples (S4 and S7) showed early ages retardation in hydration process therefore lower in their compressive strength (at 3 and 7 days), this is due to the formation of extra phase during hydration, in agreement with the XRD results shown in Fig. 3. In S4 sample an extra phases were seen in between 0 to 10 (2θ angles), this phase indicating about participation of iron sulphate during cement hydration. Similarly in S7 sample an extra peak were seen (at 2θ =11.5) which indicating about both hydrated product such as formation of Friedal’s salt (Ca3Al2O6.CaCl2.10H2O) and monocarbonate phase (21, 22, 23).
 |
Figure 3 Click here to View figure |
Conclusion
As a result of the present study, the following conclusions can be drawn:
- The additive, tin chloride was found to be the best reducing agent at dosage levels i.e., 1.0% (w/w) for reducing 25 ppm Cr (VI) along with iron sulphate additives studied (in crystalline form). It seems that the efficiency of reducing additives in their crystalline form is significantly less pronounced.
- The XRD studies indicate that the formation of extra phase (monocarbonate as well as Friedal’s salt) in the presence of tin chloride (1% w/w) is particularly visible (XRD); Friedal’s salt is responsible for delay in early hydration but monocarbonate formation is indicating about timely hydration at 28 days.
- The XRD studies indicate that the formation of extra phases at 2θ angle (b/w 0 to 9.0) is well visible in case of iron sulphate (1% w/w); extra phases are responsible for retardation in hydration process therefore lower in compressive strength. It seems that the iron sulphate acts as a strong retarder than tin chloride.
- The TGA studies indicate that the generation of Ca(OH)2 in hydrated cement with iron sulphate and tin chloride (1% w/w) was lower from hydrated Portland cement.
- The SEM studies indicate that cement sample with 1% tin salt, showed that the formation of rigid surface as well as crystal of hydrated tricalcium silicate phase. These observations well coincide with the compressive strength results.
Acknowledgement
The authors wish to thanks Dr. N. K. Katyal (Ex Incharge “Analytical Lab Service” National Council for Cement and Building Materials, Ballabgarh (Haryana), India for providing the facilities for analysis of cement samples.
References
- Davan, A.D.; Paine, A.J. Hum & Exp. Toxico. 2001, 20, 439 –451
- The Scientific Committee on toxicity, Ecotoxicity and the Environment, Risk to health from chromium hexavalent in cement, CSTEE, 2002.
- Jardine L. A.; Cornman C. R.; Gupta V.; Chun B. C. Cement Composition having Chromium Reducer, U.S. Patent 7232483, June 19, 2007.
- Larsen, Soren B.; Cement Composition and Methods for Producing Same, U.S. Patent 5362321, November 8, 1994.Cambria F.; Orlandi A.; Lanza R.; Cambi S. Process for preparing cement with a low hexavalent chromium content, Euro. Patent 1580174, February 21, 2007.
- Fiorucci.; Use of Selected Catalyzed Hydrazine Compositions to Reduce Hexavalent Chromium, U. S. Patent 4367213, January 4, 1983.
- Jardine L. A.; Amine-Based Hexavalent Chromium Reducing Agents for Cement, U.S. Patent 6872247, March 29, 2005.
- Jardine L. A.; Sulfate dispersion chromate reducer, U.S. Patent 7128782, October 31, 2006.
- Luigi C.; Castaldi.; Graziano.; Corazza.; Fabio.; Chromate Free Cements and a Process for Preparing them, Euro. Patent 697380, December 5, 2001.
- Vaity R.; Verma J. Advances in Cement Research, 2011.
- Hills, L.; Johansen, V. Portland cement Association, Skokie, IL, 2007
- Hill. J.; Sharp. J. H. Cement and Concrete Research 2003, 33, 121–124.
- S. Rekha.; S. Devesh K.; Katyal, N.K. IJOART, 2014, 3 (2), 1-5.
- Bose, M. Analytica Chimica Acta, 10 (1954) 201–208.
- Pflaum, R, T.; Howick, L, C. American Chemical Society, 1956, 78 (19), 4862–4866.
- Erdem, E.; Donat, R.; Tunc, T. E. Journal Ceramics Silikáty, 2011, 55 (1), 85-93.
- Venkateswara, V. Reddy; Research journal of chemical sciences, 2011, 1 (2), 103-107
- Suthatip, S.; Prayoon, S. Journal of Hazardous Materials, 2011, 191, 296–305
- Lothenbach, B.; Scrivener.; K. Cement and Concrete Research, 2010, 41(3), 217-229
- Gabrovšeka, R.; Vukb. Acta Chim. Slov. 2006, 53, 159–165
- Weast. R.C, Handbook of Chemistry and Physics, CRC Press, 61th edition, 1981
- Florian. Deschner.; Frank. Winnefeld.; Barbara. Lothenbach.; Sebastian. Seufert.; Peter. Schwesig.; Sebastian. Dittrich.; Friedlinde. Goetz-Neunhoeffer.; Jürgen Neubauer. Cement and Concrete Research, 2012, 42, 1389–1400
- Florian. Deschner.; Barbara. Lothenbach.; Frank. Winnefeld.; Jürgen. Neubauer. Cement and Concrete Research, 2013, 52, 169–181.
- Monica Adriana Trezza, Materials Research, 2007, 10 (4), 331-334

This work is licensed under a Creative Commons Attribution 4.0 International License.









