Density Functional theory Study of 2,1,3-Benzoxadiazole-5-carboxylic acid as photosensitizers for dye-sensitized solar cells
V. Sathyanarayanamoorthi*1, J. Prakash Manuel Joe2 and S. Mohan Kumar2
1Department of Physics, PSG College of Arts and Science, Coimbatore – 641 014, India. 2Department of Electronics, PSG College of Arts and Science, Coimbatore – 641 014, India.
DOI : http://dx.doi.org/10.13005/ojc/310116
Article Received on :
Article Accepted on :
Article Published : 16 Mar 2015
Theoretical analysis of the 2,1,3-Benzoxadiazole-5-carboxylic acid dye molecule and new designed dyes were performed using Density Functional Theory. The ground state and excited state oxidation potential as well as electron injection from dyes to TiO2 are reported. Improved light harvesting efficiency and free energy change of electron injection of new designed sensitizers reveal that these materials would be excellent sensitizers. This theoretical designing will pave way for the experimental list to synthesis the efficient sensitizers for solar cells.
KEYWORDS:DSSC; LHE; DFT; 2; 1; 3-Benzoxadiazole-5-carboxylic acid
Download this article as:| Copy the following to cite this article: Sathyanarayanamoorthi V, Joe J. P. M, Kumar S. M. Density Functional theory Study of 2,1,3-Benzoxadiazole-5-carboxylic acid as photosensitizers for dye-sensitized solar cells. Orient J Chem 2015;31(1). |
| Copy the following to cite this URL: Sathyanarayanamoorthi V, Joe J. P. M, Kumar S. M. Density Functional theory Study of 2,1,3-Benzoxadiazole-5-carboxylic acid as photosensitizers for dye-sensitized solar cells. Orient J Chem 2015;31(1). Available from: http://www.orientjchem.org/?p=7768 |
Introduction
The direct conversion of sunlight into electrical energy by solar cells is of particular interest because it has many advantages over most presently used electrical power generation methods. In recent years dye-sensitized solar cells (DSSCs), as a novel technology for the conversion of solar energy into electricity, have attracted tremendous and continuous research interest because of easy fabrication, lower cost and relatively higher efficiency compared to other photo voltaic technology. It has been found that all of the main components in DSSCs, including dye sensitizers, anode and cathode as well as electrolyte can affect the photon-to-current conversion efficiency (PCE). Specifically, the dye sensitizers, which have the function of light harvesting and photon exited electron injection, have a significant influence on the PCE.[1-5].
Due to the global challenge in searching and developing renewable energy sources, photo voltaic technologies become a topic of interest in the design of solar to electrical energy conversion cell. Even though multi junction solar cell has been successfully performed in the laboratory scale with up to 40% conversion efficiency, but their high production cost and toxicity to environment are problem to use as solar electricity in a very large scale. The feasibility to develop a solar cell, organic molecules and polymers are two of the most important alternatives.[6].
Currently, molecular modeling techniques and especially quantum chemistry offer a competitive alternative for the interpretation of the experimental data arising from industrial interests and developments. For UV/Vis calculations, one of the most popular approach remains in the time-dependent density functional theory (TDDFT),which commonly provides accurate results for a reasonable computational effort, especially when hybrid functional are used.
Therefore, we have set up a general TDDFT-based theoretical procedure for the UV/Vis prediction of BCA absorption spectra, and we strive for the 0.1-0.2 eV accuracy that is the generally accepted upper limit required for the design of new industrial dyes. To meet this target, our computational procedure is taking into account the solvation process of the dyes by means of the Polarizable Continuum Model (PCM). This above mentioned procedure is also used to gain insights into the geometrical and electronic structures, of the dyes and to bring up the adequate structural modifications to optimize the properties of the newly designed DSSCs. More precisely, in this contribution, we focus on the free energy of the electron injection onto the TiO2 substrate and on the light harvesting abilities of the dyes.[7-8]
The 2,1,3 Benzoxadiazole-5-carboxylic acid [BCA] belongs to heterocyclic building blocks and also laser dyes. It is widely used in organic electronics and photonics. The molecular formula of the chemical is C7H4N2O3. It is a hydrogen bond acceptor and donor. As such, we consider it important to understand the similarity between their properties and hope it can be used for DSSC.
Computational Details
All calculations have been performed with the Gaussian 09 package[9].The ground state geometries of 2,1,3 Benzoxadiazole-5-carboxylic acid dyes optimized with DFT at B3LYP/6-311+G(d,p) level[10,11]. The solvation effects were evaluated using Polarizable Continuum Model (PCM). [12,13]. In PCM, one usually divides the problem into a solute part (the dye) lying inside a cavity and a solvent part.[14-16].
Results and Discussion
Electronic Structure
This study was carried out to design new sensitizers for DSSC application. The designed dyes consist of following parts: Auxiliary donor (AD), donor (D), pi-spacer (π) and acceptor (A) as shown in Figure 1. We have designed new dyes by the structural modification of 2,1,3 Benzoxadiazole-5-carboxylic acid(BCA). Structure of 2,1,3 Benzoxadiazole-5-carboxylic acid(BCA) and its structural modifications are given in Figure 2. The ground state structure for 2,1,3 Benzoxadiazole-5-carboxylic acid(BCA) dyes were carried out at the B3LYP- 6-311+G(d,p) level. The discussion based on geometrical parameter of ground state structure is neglected. The highest occupied molecular orbital energies (HOMO) and the lowest unoccupied molecular orbital energies LUMO of all BCA dyes computed at the B3LYP-6311+G(d,p) level in gas phase and DMF are listed in table 1. Both HOMO and LUMO levels for all dyes in solvent medium are higher than that of a gas phase. The energy gap also decreased in DMF except BCA-4. This may be due to the environmental media playing a major role in stabilization of HOMO. However, the least positive value of energy gap in figure 3 shows that the data of HOMO, LUMO energies was extracted from optimization output in gas phase. Figure 4 show the levels of HOMO, LUMO of all the dyes in solvent phase when the frontier molecular orbital of all dyes are compared with the corresponding metal oxide. To get further insights into the molecular structure and electronic distribution of these organic dyes, we have performed the molecular orbital analysis (MOA) at the B3LYP/6-311+G(d,p) level of theory. The electron distribution for the HOMO and LUMO of BCA derivatives are depicted in figure 5. For all the considered systems the HOMO is essentially localized on the nitrogen atoms whereas LUMO is located in anchoring group through π-bridge. The MOA reveals that the canon-acrylic acid group is essentially coplanar to the phenyl group. It is also important to underline that in view of the MOA, the HOMO→LUMO excitation induced by light irradiation could move the electron distribution from the BCA moiety to the anchoring group. Therefore, assuming a similar molecular orbital shape when the dye is anchored to the LUMO centered on the anchoring moiety should enhance the orbital overlap with the titanium 3d orbital and subsequently favour the electron injection in TiO2 matrix.
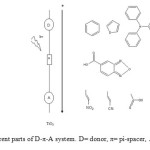 |
Figure1: Different parts of D-π-A system. D= donor, π= pi-spacer, A= acceptor. Click here to View figure |
![Figure2 Chemical Structure of 2,1,3 Benzoxadiazole-5-carboxylic acid [ BCA] newly designed dyes](http://www.orientjchem.org/wp-content/uploads/2015/03/Vol31_No1_Dens_Sathya_Fig2-150x150.jpg) |
Figure2: Chemical Structure of 2,1,3 Benzoxadiazole-5-carboxylic acid [ BCA] newly designed dyes Click here to View figure |
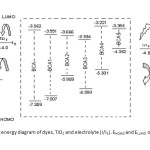 |
Figure3: Schematic energy diagram of dyes, TiO2 and electrolyte (I/I3). EHOMO and ELUMO of the dyes are in gas phase Click here to View figure |
 |
Figure4: Schematic energy diagram of dyes, TiO2 and electrolyte (I/I3). EHOMO and ELUMO of the dyes are in DMF Click here to View figure |
 |
Figure5: The HOMO and LUMO distribution pattern of dyes at B3LYP/6-311+G level of theory Click here to View figure |
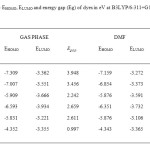 |
Table1: The EHOMO, ELUMO and energy gap (Eg) of dyes in eV at B3LYP/6-311+G level of theory Click here to View table |
Electron Injection
In this section, we focus on the evaluation of the electro chemical properties of the dyes in their excited state. More precisely, we propose to establish a reliable theoretical scheme to quantify the electron injection onto a Titanium Dioxide (TiO2) surface for selected BCA derivatives. [17-19]
The free energy change (in eV) for the electron injection can be expressed as
![]()
Where EdyeOX is the oxidation potential of the dye in the excited state, and E SCCB is the reduction potential of the conduction band of the semiconductor. It is often difficult to accurately determine E SCCB experimentally because it is sensitive to the surface conditions, as well as to the pH of the solution. Nevertheless, the E SCCB values of several materials have been reported, and have been using E SCCB = 4.0 eV for TiO2 .The presented value has been experimentally determined and refers to conditions where the semiconductor is in contact with aqueous redox electrolytes of fixed pH.
In table 2, we provide the electron injection free energy (ΔGinject), and ground [Eoxdye] excited [Eoxdye*] state oxidation potential computed in gas phase and solvent phases for the series of BCA dyes. From these results one can conclude that the effects of the dielectric surroundings on ΔGinject value is huge with the shift of approximately -0.2 eV when going from gas to DMF. Note that the shift of the both ground and excited state oxidation potential in solvent phase reduce which results in the stabilization of oxidized dyes and favour the electron injection in solvent medium. A comparison between gas and solvent phase oxidation potential allows to conclude that the ground state oxidation potential cathodic shift induced by the solvent is only loosely sensitive to the permittivity of the surroundings. All ΔGinject estimated in the table 2 is a negative value for all dyes. More negative of ΔGinject from gas to solvent medium were found. From these results , it is indicated that the effect of dielectric surroundings to ΔGinject values is huge with a shift of -0.18 eV from gas to solvent.
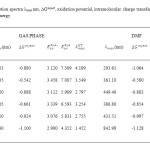 |
Table2: Calculated absorption spectra λmax nm, ∆Ginject, oxidation potential, intramolecular charge transfer energy of dyes at B3LYP/6-311+G level of energy Click here to View table |
Light Harvesting Efficiency (Lhe) and Oscillator Strength
In the present section, we propose structural modifications improving the electron injection efficiency of the BCA based DSSCs. Of course, all modifications are theoretically possible and a large panel of new structure can be tested. We focus on three properties that can be optimized (i) the free energy of injection ΔGinject. (ii) the oxidation potential of the dyes must be more positive, (iii) the light harvesting efficiency (LHE) of the dye has to be as high as possible to maximize the photocurrent response. More precisely, LHE is expressed as[20]
LHE=1-10-A=1-10-f
Where A(f) is the absorption (oscillator strength) of the dye associated to the λmax .It is known that TDDFT is less efficient for the evaluation of transition probabilities than for transition energies. For the sake of computational consistency, the LHE criterion has therefore to be under weighted in our classification, as our estimates of ΔGinject and are probably more reliable .However, we have to underline that the dependence of the experimental extinction coefficient with respect to auxochromic effects was qualitatively reproduced for the 2,1,3 Benzoxadiazole-5-Carboxylic acid derivatives.
The light harvesting efficiency (LHE) is the efficiency of dye in responding to light .It is another factor which indicates the efficiency of DSSC. The light harvesting efficiency (LHE) of the dye should be as high as possible to maximize the photocurrent response. The value of LHE of the dyes has to be as high as possible to maximize the photocurrent response. The LHE has to be underweighted for the calculation. These values are important for charge transfer process in DSSC. So the LHE is regarded to underweight in this work. The LHE of all the dyes are calculated and listed in table 3. The LHE of all the dyes fall within the range of 0.007-0.166 in gas phase and 0.036-0.295 in gas to DMF. The LHE values for the dyes are in a narrow range.
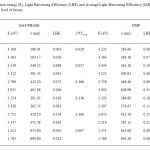 |
Table3: Excitation energy (E), Light Harvesting Efficiency (LHE) and Average Light Harvesting Efficiency (LHFAverage) of dyes at B3LYP/6-311+G level of theory Click here to View table |
This means that all the dyes will give similar photocurrent. It can be concluded that a class of selected BCA derivative dyes shows a good photo physical properties related to DSSC use but in different outstanding properties. According to LHE, BCA3 in gas phase and BCA2 in DMF phase are the most efficient than that of the other derivatives studied here. From this we can assume that substitutes of acceptor and donor atoms can enrich the properties of BCA derivatives dyes for the use in DSSC.
The oscillator strength is directly obtained from DFT calculations. The BCA dye has two main absorption peaks [355 and 296 nm in gas phase, 293 and 284 nm in solvent phase DMF].Higher oscillator strength of the four new designed sensitizers than that of BCA is as shown in table 3.These dyes will convert more light to electrical energy. The oscillator strength and transition character are given in table 4.Only the transition with considerable oscillator strengths are given. The electron structure of the four new designed sensitizers is quite similar to one another. Improve ΔGinject and LHE of new designed sensitizers as compared to BCA is due to the addition of acceptor and donor molecules. In the benzene ring this can be considerable by the distribution pattern of HOMO and LUMO of BCA derivatives. It encourages the promotion of electron injection.
Electronic Absorption Spectra
TD/DFT/6-311+G(d,p) methods were employed to simulate the optical properties of the dyes. The computed vertical excited singlet states, transition energies and oscillating strength of all dyes in gas phase and solvent medium are tabulated in table 4.The graph plotted wavelength versus oscillator strength are shown in figure 6.
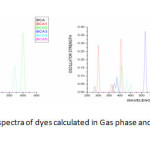 |
Figure6: Simulated absorption spectra of dyes calculated in Gas phase and DMF at B3LYP/6-311+G level of theory Click here to View figure |
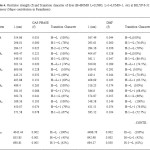 |
Table4: Oscillator strength (f) and Transition character of dyes (H=HOMO L=LUMO, L+1=LUMO+1, etc) at B3LYP/6-311+G level of theory (Major contribution in Paranthesis) Click here to View table |
The vertical excitation energies of the dyes are increasing ΔEexi its value by the substitution. The transition to the character of most of the dyes show the HOMO, LUMO transition as the first singlet excitation except for BCA. In the major contribution of the transition characters in the gas phase differs from those solvent due to the effect of polar environment and the electronic energy level. The maximum wavelength for UV-Vis absorption spectra of all the dyes simulated in various media are shown in table 4.The calculation in gas phase and solvent medium is different by approximately the red shift were found for most of the dyes from gas phase and solvent phase.
Conclusion
This work is reported as a theoretical study of 2,1,3 Benzoxadiazole-5-carboxylic acid dye using DFT method. The ground and the excited state oxidation potential energy as well as electron injection of the dyes show that these dyes are potential to be good photo sensitizers in DSSC. The LUMO of the dyes with nearly 1.0 eV upper than Ecb of TiO2 indicated that these dyes are thermodynamically favorable in charge transfer into conduction band of TiO2. All new designed dyes were highly red shifted as compared to 2,1,3 Benzoxadiazole-5-carboxylic acid due to solvent effect. It can be noted that on the BSC dyes in this calculations give the better DSSC efficiency. However we suggest that the further chemical modification of the dye such as adding high effective electron acceptors and donors could raise the ∆Ginject and LHE of the DSSC with these photo sensitizers.
References
- Austin Suthanthiraraj.S,Indian Journal of Pune and applied physics 2013., 51.,310-314.
- Francisco Cervantes-Navarro, Daniel Glossman-Mitnik, Journal of Photochemistry and Photobiology A: Chemistry 2013., 255 ., 24-26.
- Kamat. P.V, Haria.M, Hotchandani.S, J. Phys. Chem. 2004., 108,.5166.
- Bisquert.J, Cahen.D, Hodes.G, Ruehie.S, Zaban.A, J. Phys. Chem. 2004.,108 8106.
- Furube.A, Katoh.R, Yoshihara.T, Hara.K, Murata.S, Arakawa.H, Tachiya.M, J. Phys. Chem.. 2004 .,108 .,12588.
- Gratzel.M, Recent advances in sensitized mesoscopic Solar Cells, Acc. Chem. Res. 2009 .,42 1788-1798.
- Serrann.L – Andres, Roos.B.O, A theoretical study of the indigoid dyes and their chromophore, Chemistry – A European Journal 1997 ., 3 .,717-725.
- Susse.P, Steins.M, V. Indigo Crystal Structure refinement based on synchrotron data, Zeitschrift fur Kristallograhie 1988 .,184 269-273.
- Gaussian 09 Program, Revision C.01,. Frisch.J, Trucks.G.W, .Schlegel.H.B, Scuseria.G.E, Robb.M.A, Cheeseman.J.R, Scalmani.G, Barone.V, Mennucci.B, Petersson.G.A, Nakatsuji.H, Caricato.M, Li.X, Hratchian.H.P, Izmaylov.A.F, Bloino.J, Zheng.G, Sonnenberg.J.L, Hada.M, Ehara.M, Toyota.K, Fukuda.R, Hasegawa.J, Ishida.M, Nakajima.T, Honda.Y, Kitao.O, Nakai.H, Vreven.T, Montgomery.J.A, Jr., Peralta.J.E, Ogliaro.F, Bearpark.M, Heyd.J.J, Brothers.E, Kudin.K.N, Staroverov.V.N, Keith.T, Kobayashi.R, Normand.J, Raghavachari.K, Rendell.A, Burant.J.C, Iyengar.S.S, Tomasi.J, Cossi.M, Rega.N, Millam.J.M, Klene.M, Knox.J.E, Cross.J.B, Bakken.V, Adamo.C, Jaramillo.J, Gomperts.R, Stratmann.R.E, Yazyev.O, Austin.A.J, Cammi.R, Pomelli.C, Ochterski.J.W, Martin.R.L, Morokuma.K, Zakrzewski.V.G, Voth.G.A, Salvador.P, Dannenberg.J.J, Dapprich.S, Daniels.A.D, Farkas.O, Foresman.J.B, Ortiz.J.V, Cioslowski.J, and Fox.D.J, Gaussian, Inc., Wallingford CT, 2010.
References
- Austin Suthanthiraraj.S,Indian Journal of Pune and applied physics 2013., 51.,310-314.
- Francisco Cervantes-Navarro, Daniel Glossman-Mitnik, Journal of Photochemistry and Photobiology A: Chemistry 2013., 255 ., 24-26.
- Kamat. P.V, Haria.M, Hotchandani.S, J. Phys. Chem. 2004., 108,.5166.
- Bisuert.J, Cahen.D, Hodes.G, Ruehie.S, Zaban.A, J. Phys. Chem. 2004.,108 8106.
- Furube.A, Katoh.R, Yoshihara.T, Hara.K, Murata.S, Arakawa.H, Tachiya.M, J. Phys. Chem.. 2004 .,108 .,12588.
- Gratzel.M, Recent advances in sensitized mesoscopic Solar Cells, Acc. Chem. Res. 2009 .,42 1788-1798.
- Serrann.L – Andres, Roos.B.O, A theoretical study of the indigoid dyes and their chromophore, Chemistry – A European Journal 1997 ., 3 .,717-725.
- Susse.P, Steins.M, V. Indigo Crystal Structure refinement based on synchrotron data, Zeitschrift fur Kristallograhie 1988 .,184 269-273.
- Gaussian 09 Program, Revision C.01,. Frisch.J, Trucks.G.W, .Schlegel.H.B, Scuseria.G.E, Robb.M.A, Cheeseman.J.R, Scalmani.G, Barone.V, Mennucci.B, Petersson.G.A, Nakatsuji.H, Caricato.M, Li.X, Hratchian.H.P, Izmaylov.A.F, Bloino.J, Zheng.G, Sonnenberg.J.L, Hada.M, Ehara.M, Toyota.K, Fukuda.R, Hasegawa.J, Ishida.M, Nakajima.T, Honda.Y, Kitao.O, Nakai.H, Vreven.T, Montgomery.J.A, Jr., Peralta.J.E, Ogliaro.F, Bearpark.M, Heyd.J.J, Brothers.E, Kudin.K.N, Staroverov.V.N, Keith.T, Kobayashi.R, Normand.J, Raghavachari.K, Rendell.A, Burant.J.C, Iyengar.S.S, Tomasi.J, Cossi.M, Rega.N, Millam.J.M, Klene.M, Knox.J.E, Cross.J.B, Bakken.V, Adamo.C, Jaramillo.J, Gomperts.R, Stratmann.R.E, Yazyev.O, Austin.A.J, Cammi.R, Pomelli.C, Ochterski.J.W, Martin.R.L, Morokuma.K, Zakrzewski.V.G, Voth.G.A, Salvador.P, Dannenberg.J.J, Dapprich.S, Daniels.A.D, Farkas.O, Foresman.J.B, Ortiz.J.V, Cioslowski.J, and Fox.D.J, Gaussian, Inc., Wallingford CT, 2010.
- Becke.A.D, J. Chem. Phys. 1993., 98 .,1372.
- Lee.C, Yang.W, Parr.R.G, Phys. Rev. B .,1988.,37., 785.
- Tomsi.J, Mennucci.B, Cances.E.T, J. Mol. Structure THEOCHEM. . 1999,464 .,211.
- Cossi.M, Barone.V, J. Chem. Phys. . 1998,109 .,6264.
- Cossi.M, Barone.V, J. Chem. Phys. 2001., 115 4708.
- Amovilli.C, Barone.V, Cammi.R, Cances.E, Cossi.M, Mennucci.B, Pomelli.C.S, Tomasi.J, Adv. Quantum Chem. .,1998.,32., 227.
- Tomasi.J, Mennucci.B, Cammi.R, Chem. Rev. 2005., 105 .,2999.
- Kotob.R, Furube.A, Yoshihara.T, Hara.K, Fujihashi.G, Takano.S, Murata.S, Arakawa.H, Tachiya.M, J. Phys. Chem. B 2004, 108. 4818.
- Rehm.D, Weller.A, Isr. J. Chem. 1970 .,8 .,259.
- Goodman.J.I, Peters.K.S, J. Am. Chem. Soc. 1986 .,108 .,1700.
- Nalwa .H.S, Handbook of Advanced Electronic and Photonic Materials and Devices; Academic: San Diego. (2001).Becke.A.D, J. Chem. Phys. 1993., 98 .,1372.
- Lee.C, Yang.W, Parr.R.G, Phys. Rev. B .,1988.,37., 785.
- Tomsi.J, Mennucci.B, Cances.E.T, J. Mol. Structure THEOCHEM. . 1999,464 .,211.
- Cossi.M, Barone.V, J. Chem. Phys. . 1998,109 .,6264.
- Cossi.M, Barone.V, J. Chem. Phys. 2001., 115 4708.
- Amovilli.C, Barone.V, Cammi.R, Cances.E, Cossi.M, Mennucci.B, Pomelli.C.S, Tomasi.J, Adv. Quantum Chem. .,1998.,32., 227.
- Tomasi.J, Mennucci.B, Cammi.R, Chem. Rev. 2005., 105 .,2999.
- Kotob.R, Furube.A, Yoshihara.T, Hara.K, Fujihashi.G, Takano.S, Murata.S, Arakawa.H, Tachiya.M, J. Phys. Chem. B 2004, 108. 4818.
- Rehm.D, Weller.A, Isr. J. Chem. 1970 .,8 .,259.
- Goodman.J.I, Peters.K.S, J. Am. Chem. Soc. 1986 .,108 .,1700.
- Nalwa .H.S, Handbook of Advanced Electronic and Photonic Materials and Devices; Academic: San Diego. (2001).

This work is licensed under a Creative Commons Attribution 4.0 International License.









