Bioremediation of heavy metals by employing resistant microbial isolates from agricultural soil irrigated with Industrial Waste water
Vijay Kumar1, Simranjeet Singh2, Nivedita Kashyap2, Sourav Singla2, Pooja Bhadrecha2, Parvinder Kaur2, Shivika Datta3, Arjun Kalia 2,Joginder Singh2*
1Department of chemistry, Lovely Professional University, Phagwara, Punjab.
2*Department of Biotechnology, Lovely Professional University,Phagwara, Punjab.
3Department of Zoology, Lovely Professional University, Phagwara, Punjab.
DOI : http://dx.doi.org/10.13005/ojc/310142
Article Received on :
Article Accepted on :
Article Published : 24 Feb 2015
This study was focused to isolate the most efficient bacteria from heavy metal contaminated soil from Ludhiana, Punjab. A total of 14 microbial isolates were characterized and out of 14, IS1 and IS14 were observed to be most effective because of their high relative growth and resistance against heavy metals. Further, these two isolates were assessed for their ability to remove Zinc and Lead from medium amended with heavy metals. IS1, Bacillus thuringiensis strain “Simi” (Accession number KF 916618.1) was found to be more effective as compared to IS14, Bacillus subtilis strain PSB (Accession number KF 279045.1) for the remediation of heavy metals. IS1 showed mean of 54% biodegradation efficacy in the first three days and from day 4 on wards the mean percentage of biodegradation efficacy decreased to around 31%. The results of the present study showed that the metal resistant bacteria can be used for heavy metal bio accumulation.
KEYWORDS:CP-AES; Phylogenetic tree; waste water; Hydrocarbons
Download this article as:| Copy the following to cite this article: Kumar V, Singh S, Kashyap N, Singla S, Bhadrecha P, Kaur P, Datta S, Kalia A, Singh J. Bioremediation of heavy metals by employing resistant microbial isolates from agricultural soil irrigated with Industrial Waste water. Orient J Chem 2015;31(1). |
| Copy the following to cite this URL: Kumar V, Singh S, Kashyap N, Singla S, Bhadrecha P, Kaur P, Datta S, Kalia A, Singh J. Bioremediation of heavy metals by employing resistant microbial isolates from agricultural soil irrigated with Industrial Waste water. Orient J Chem 2015;31(1). Available from: http://www.orientjchem.org/?p=7422 |
Introduction
The presence of heavy metals and pesticides in the environment has been a subject of great concern due to their toxicity, non-biodegradable nature and the long biological half-lives for their elimination from biological tissues1-5. Pollutants deteriorates the quality of soil and crops produced. Excessive metal concentrations and pesticides in contaminated soils can result in decreased soil microbial activity and soil fertility, and yield losses2-3. Agricultural irrigation with wastewater is common in arid areas but has possible public health and environmental side effects, as effluent may contain pathogens, high level of salts, detergents and toxic metals2-5.
There is a need for monitoring of toxic effects of wastewaters and such irrigation practices should be carried out only after treatment of wastewater. Numerous methods have been proposed to remove heavy metals from sewage sludge, including chlorination, use of chelating agents and acid treatments at high temperatures 6-7. However, those methods are generally ineffective in practical applications due to high cost, operational difficulties and low metal leaching efficiency. An alternative way to replace chemical methods in removing heavy metals is bioremediation through microbial isolates.
The bioremediation techniques are effective and efficient for remediation of pollutants so as the bioremediation technology from laboratory to field to clean up the environment can be taken up8. For bioremediation to be effective, microorganisms must enzymatic ally attack the pollutants and convert them to harmless products9. As bioremediation can be effective only where environmental conditions permit microbial growth and activity, its application often involves the manipulation of environmental parameters to allow microbial growth and degradation to proceed at a faster rate. These factors include the existence of a microbial population capable of degrading the pollutants; the availability of contaminants to the microbial population; the environment factors10.Therefore, this study was designed with the objective to isolate and characterize metal (Zinc and lead) resistant bacteria from heavy metal contaminated soil to determine their feasibility on the removal of metals through bio-accumulation.
Methodology
Collection and Physicochemical Analysis of Soil Samples
Soil samples were collected from the top 15 cm from industrial effluents of the Ludhiana (Punjab) region (30. 91o N 75. 85o E). These samples were collected in sterile zip lock bags with the help of sterilized spatula, properly sealed and labeled, and sent to the laboratory within 24 hours. Heavy metals in soil were analyzed by inductively coupled plasma-atomic emission spectrometry at P.A.U., Ludhiana (ICP-AES).
Isolation & Identification of the Micro organism
Isolation was done by the method as described by Jyothi11. Isolated microbes were identified through morphological, biochemical& molecular characterization12.
Determination of Comparative Growth and Growth Pattern
Extent of heavy metal resistance of selected microbial isolates was evaluated in bacillus cereus broth (bacteria) containing 25, 100, 250 and 300 ppm of Lead nitrate Pb(NO3)2, Copper sulphate CuSO4, Zinc nitrate Zn(NO3)2 and Ferrous sulphate FeSO4 and growth is determined by measuring Optical density (O.D.)at 540 nm with un-inoculated broth as control. To check the growth pattern of the isolates, cultures were inoculated into broth, treated with 0 (control), 25, 100, 250 and 300 ppm of Pb(NO3)2, CuSO4, Zn(NO3)2 and FeSO4, and incubated at 37oC13.
Molecular Analysis
Isolates showing high resistant against heavy metals were sent for 16 S r RNA analysis to Ahmedabad, Gujarat for molecular analysis14-15.
Minimum Inhibitory Concentration (MIC) Determination of the Isolated Strains
Bacillus cereus agar plates and yeast pep tone dextrose agar plates were inoculated with 100µl aliquots of 24 hr culture with all isolates with different concentrations of Pb(NO3)2, CuSO4, Zn(NO3)2 and FeSO4 (250, 500,750,1000,1250 and 1500 mg/l).Diameter of inhibition zones were measured (in mm) in order to determine the MIC16.
Efficacy of Isolated Strains on Bioaccumulation of Heavy metals
80µl of the bacterial inoculum was inoculated into test tubes containing 8.0 mL of broth supplemented with 100 ppm of zinc & lead. The control treatment was prepared by mixing 9.0 ml of broth with 1.0 ml of bacterial suspension. All the tubes were sealed with para film and kept at 27 ± 2 °C for 7 days. To determine degradation efficacy, the heavy metal was first extracted with sequential extraction method17 diluted 10-4 times and the absorbance was recorded at 225 nm18. The biodegradation efficacy (BE) was calculated using the following formula BE (%) = 100 – (As/Aac × 100)19,20.
Results & Discussion
Soil Analysis Report and Enumeration of Bacteria
Soil analysis report shows the presence of various toxic heavy metals and sample contains Arsenic(0.756 mg/kg), Calcium (327.9 mg/kg), Cadmium (0.09 mg/kg), Cobalt (0.02 mg/kg), Chromium (0.053 mg/kg), Copper (9.4 mg/kg), Iron (53.71 mg/kg), Potassium (118.6 mg/kg), Magnesium (202.7 mg/kg), Manganese (4.539 mg/kg), Sodium (59.91 mg/kg), Nickel (0.723 mg/kg), Phosphorous (65.02 mg/kg), Lead (8.387 mg/kg), and Zinc (10.8 mg/kg). A total of fourteen different isolates (IS1,IS2, IS3, IS4, IS5, IS6, IS7, IS8, IS9, IS10, IS11, IS12, IS13, and IS14) were isolated and are biochemically and molecularly characterized and best two having high resistant against metals were further selected for bio accumulation & heavy metal testi.e. IS1 and IS 14. The biochemical and isolated results were shown in fig. 1 and table 1.
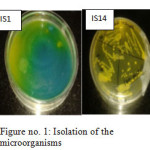 |
Figure1: Isolation of the microorganisms Click here to View figure |
| Test | IS1 | IS14 |
| Indole test |
– |
– |
| Methyl red |
+ |
+ |
| VogesProskauer |
– |
+ |
| Citrate |
+ |
– |
| Catalase |
+ |
+ |
| Coagulase |
– |
+ |
| Motility |
+ |
– |
| Nitrate reduction |
+ |
+ |
Table no1: Biochemical tests:
Relative growth determination of isolates and their growth pattern
It was observed that the growth of the isolate decreases with the increase in metal concentration.IS1 Bacillus thuringiensis strain Simi and IS14 was found to have maximum relative growth against Lead and Zinc solution. The relative growth rate was observed at different concentration of heavy metals. The results are consisted with Ahemad and Malik15. The growth pattern of the microbial isolate sat different concentration(25,100,250&300 µgml-1)are shown below:
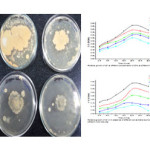 |
Figure2: Isolates amended with different concentrations of metals & Graph showing relative growth of isolates at different concentrations (Control, 25 ppm, 100 ppm 200 ppm, 300 ppm of metal solution) of Zinc and Lead. Click here to View figure |
Heavy metal tolerance test
All the isolates were tested for heavy metal tolerance test against Pb(NO3)2, and Zn (NO3)2. The IS1 exhibited maximum tolerance for zinc and IS14 exhibited maximum tolerance for lead and the results are shown in figure 3.
Heavy metal tolerance test for IS1 & IS14
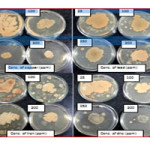 |
Figure 3Click here to View figure |
16 sr RNA Sequence Analysis
The isolates IS1 and IS14 were found similar to Bacillus thuringiensis strainsimi & Bacillus subtilis under accession number KF 916618.1 & KJ 489411.1, when submitted in NCBI. The phylogenetic tree shows relationship of isolated strains with other species.
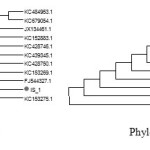 |
Figure 4Click here to View figure |
Determination of Minimum Inhibitory Concentration (MIC)
A great deal of variation among the isolates against the different heavy metals was observed.The results in Table 8 reveal that MIC of Zinc for Bacillus thuringiensis strain Simi was at 1000 µg/ml where as Bacillus subtiliss train PSB had maximum MIC for lead.
| Metals | IS1 | IS14 |
| Zinc | 960±10 | 100±10 |
| Copper | 100±10 | 500±10 |
| Lead | 250±10 | 1000±10 |
| Iron | 250±10 | 500±10 |
Table 2 Minimum Inhibitory Concentration (µgmL-1).
Biodegradation Efficacyon Heavy Metals
The degradation efficacy of Bacillus thuringiensis strain Simionzinc showed rapid degradation in the first three days, with mean of 54% biodegradation efficacy after fourth day the degradation efficacy decreased and reached to the point of 31% and Bacillus subtilis strain PSB showed less degradation activity but the decrease in degradation efficacy started after fifth day. Similar work has already been reported against the hydrocarbon degradation by the isolate P. lundensis UTAR FPE218.
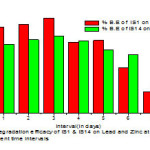 |
Figure 5Click here to View figure |
Efficacy of IS1 and IS14 on Biodegradation of lead and Zinc solution.
Conclusion
The removal of heavy metals or break down into harmless state has become necessary. Thus bioremediation can be employed for the removal of such contaminants. This study of Zinc and lead accumulation by the isolates from heavy metal contaminated soils revealed a good and positive sign for its further use in bioremediation of zinc and lead in contaminated sites. The current study has illustrated some basic considerations that are important for the use of metal accumulating bacteria for bioremediation under field conditions.
References
- Kumar, V.; Upadhyay, N.; Wasit, A.B.; Singh, S.; Kaur, P. Curr World Environ.2013, 8(2),313-318.
- Kumar, V.; Upadhyay, N.; Singh, S.; Singh, J.; Kaur, P.Curr World Environ.2013,8, 469-473.
- Kumar, V.; Upadhyay, N.; Kumar, V.; Kaur, S.; Singh, J.; Singh, S.; Datta, S. J. Bio. Env. Sci. 2014,5, 111-120.
- Prasad, R.; Upadhyay, N.; Kumar, V. Microchem. J.2013, 111, 91-97.
- Kumar, V.;Upadhyay, N.; Kumar, V.; Sharma, S. J. Bio. Env. Sci. 2014,5, 149-165.
- Olatunji,B.O.;Deacon,B.J.; Abramo witz,J.S.Cog. Behav Sci.2009,3(2), 172-180.
- Faryal,R.;Faheem,T.;Abdul,H.Pak. J. Bot.2007,39(1),193-204.
- Hamby, D.Sci Direct.1996,191(3),203-224.
- Pandey,B.Fulekar, M.H.Biol Med. 2012,4(1),51-59.
- Mueller,J.G.Cerniglia, C.E.;Pritchard,P.H.In Bioremed Principles App.1996,3,125–194.
- Sharma,S. Asian JP harm Life Sci.2012,2(2),202-213.
- Jyothi,N.;Rao,U.V. Iran.J.Microbiol.2009,1(3),23-30.
- Aneja, K.R.Experiments in Microbiology, Plant Pathology and Biotechnology, Wishwa Prakashan New Delhi(1993).
- Ahemad,M.;Malik,A.BacteriolJ. 2012,2,12-21.
- Amann, R.I.;Ludwig,W.;Shleifer,K.H. Microbiol. Rev.1995,59,143-169.
- Pace,N.R.;Stahl, D.A.;Lane,D.J.Olsen GJ Adv. Gen. Microbiol. Ecol.1986,9,1-55.
- Murthy,S.;Bali,G.;Sarangi,S.K.IntJMicrobiolRes. 2012, 4(3),196-200.
- Emmerich,W.E.;Lund,L.J.;Page,A.L.JEnviron. Qual.1997,11, 178-192.
- Adeline,T.;Carol,S.Y.;Tan,H.C. Aw,C.Malays JMicrobiol. 2009,5(2),104-108.
- Michaud,L.;Giudice,A.L.;Saitta,M.;Domenico,M.D.;Bruni,V.MarinPollution Bull.2004,45,405-409.

This work is licensed under a Creative Commons Attribution 4.0 International License.









