Synthesis of new Guanidium-Meldrum acid zwitterionic salts and dynamic NMR study of rotational energy barrier around C-NH bond of guanidine moiety
Ali Aminkhani
Department of Chemistry, Khoy Branch, Islamic Azad University, Khoy, Iran
DOI : http://dx.doi.org/10.13005/ojc/300439
Article Received on :
Article Accepted on :
Article Published : 09 Dec 2014
Synthesis of the Guanidium-Meldrum acid zwitterionic salts was developed through a three component reaction of 2, 2-dimethyl-1, 3-dioxane-4, 6-dione (Meldrum acid), aromatic aldehydes and N, N, N', N'-Tetramethyl-guanidine in benzen at room temperature. These reaction conditions allow the preparation of stable zwitterionic salts in good yields. A dynamic NMR effect is observed as a result of restricted rotation around the C-NH bond in the 1H NMR spectra of these compounds. The free-energy of activation (∆G#) for this process is 66 ± 2 kJmol-1 for 4b.
KEYWORDS:Benzaldehydes; Dynamic NMR; Meldrum acid; Michael addition; N; N; N'; N'-Tetramethyl-guanidine; Zwitterionic salts
Download this article as:| Copy the following to cite this article: Aminkhani A. Synthesis of new Guanidium-Meldrum acid zwitterionic salts and dynamic NMR study of rotational energy barrier around C-NH bond of guanidine moiety. Orient J Chem 2014;30(4) |
| Copy the following to cite this URL: Aminkhani A. Synthesis of new Guanidium-Meldrum acid zwitterionic salts and dynamic NMR study of rotational energy barrier around C-NH bond of guanidine moiety. Available from: http://www.orientjchem.org/?p=5625 |
Intruduction
The Knoevenagel condensation of Meldrum acid with aromatic aldehydes with the aim of forming a carbon-carbon double bond is a well-documented reaction 1 that carried out using various conditions. 2 Arylidene Meldrum acids are useful reactive intermediates, being susceptible to 1, 4-addition, 3 in Diels-Alder reactions acting as activated dienophiles 4 and preparation of heterocyclic molecules such as coumarins, 5 indoles and benzofurans 6 and other useful compounds.4,7Recently we prepared an unusual charge-separated Guanidium-meldrum acid zwitterionic salts using three component reaction. The zwitterions are often as an intermediate species in some reactions. 8-10 we now describe the synthesis zwitterionic salts by means of a reaction of N, N, N’, N’-Tetramethyl-guanidine with conjugated electrophilic heterodienes. Dynamic NMR provides important kinetic data and affords good information in this matter on a dynamic process, when discussing the barrier separating two states that are observable by NMR spectroscopy. 11 Thus, herein the free-energy of activation (ΔG#) for restricted rotation around the C-NH bond of zwitterionic salt 4b is described.
Material and Methods
General
Compounds 1–3 were obtained from Fluka and Merck and were used without further purification. The following instruments were used: mp., Electrothermal-9100 apparatus, uncorrected; IR spectra, Shimadzu IR-460 spectrometer; 1H and 13C NMR spectra, Bruker DRX-300 AVANC E instrument; in CDCl3 at 300.1 MHz and 75.4 MHz, respectively, δ in ppm, J in Hz; EI-MS (70 eV): Finnigan-MAT-8430 mass spectrometer, in m/z. Elemental analyses (C, H, N) were performed with a Heraeus CHN-O-Rapid analyzer.
General procedure for preparation of Guanidium-meldrum acid zwitterionic salts (exemplified by 4b) To a magnetically stirred of 0.158 g of meldrum acid (1.1 mmol) and 0.120 g of 4-methylbenzaldehyde (1.0 mmol) in 10 cm3 of dry benzen (distilled from Na/benzophenone, 5.0 mL, 0.2 M) was added 200 µL of a 0.5 mM solution of pyrrolidinium acetate in benzene (prepared by dropwise addition of AcOH to piperidine in benzene, 0.1 mmol, 10 mol%) and stirred about 20 hour. Then the reaction mixture was washed with saturated NaHCO3 solution (2 × 10 mL). Then 0.115 g of N, N, N’, N’-Tetramethyl guanidine (1.0 mmol) in 3 cm3 of benzen was added to organic layer over one minute at room temperature. After less four hours stirring at room temperature, the solvent was removed and the crude product washed by acetone (2 × 3 mL), and residue powder filtered.
5-{((bis (dimethylamino) methylene) ammonio) (phenyl) methyl}-2, 2-dimethyl-4, 6-dioxo-1, 3-dioxin-5-ide (4a)
White powder, mp 168-170 °C (decomp), 0.236 g, (68%). IR (KBr) (νmax/cm−1): 1612 (C=O), 1681 (C=N), 3254 (NH). 1H NMR (300.1 MHz, CDCl3): δ 1.56 (6H, s, CMe2), 2.92 and 2.99 (12H, 2s, 2NMe2), 5.67 (1H, d, 3JHH = 8.6 Hz, HN-CH), 7.20 (1H, t, 3JHH = 7.4 Hz, CH), 7.28 (2H, d, 3JHH = 7.4 Hz, 2CH), 7.56 (2H, d, 3JHH = 7.4 Hz, 2CH), 9.05 (1H, d, 3JHH = 8.6 Hz, NH) ppm. 13C NMR (75.4 MHz, CDCl3): δ 26.33 (CMe2), 40.08, 40.79 (2NMe2), 57.37 (HN-CH), 77.05 (HC-C-C) 102.32 (CMe2), 126.1 (2CH), 127.4 (CH), 128.9 (2CH), 143.8 (C), 161.74 (N-C-NH), 166.66 (2C, s, 2C=O) ppm. MS, m/z (%): 347 (M+, < 1), 246 (34), 173 (100), 160 (41), 115 (79), 91 (21), 71 (52), 43 (37). Anal. Calcd for C18H25N5O3 (347.41): C, 62.23; H, 7.25; N, 12.10. Found: C, 62.18; H, 7.17; N, 12.20
5-{((bis (dimethylamino) methylene) ammonio) (p-tolyl) methyl}-2, 2-dimethyl-4, 6-dioxo-1, 3-dioxin-5-ide (4b)
White powder, mp 182-184 °C (decomp), 0.275 g, (76%). IR (KBr) (νmax/cm−1): 1608 (C=O), 1681 (C=N), 3236 (NH). 1H NMR (300.1 MHz, CDCl3): δ 1.55 (6H, s, CMe2), 2.28 (3H, s, Me), 2.92 and 2.98 (12H, 2s, 2NMe2), 5.67 (1H, d, 3JHH = 8.5 Hz, HN-CH), 7.08 (2H, d,J = 7.8 Hz, 2 CH), , 7.38 (2H, d,J = 7.8 Hz, 2CH), 8.97 (1H, d, 3JHH = 8.5 Hz, NH) ppm. 13C NMR (75.4 MHz, CDCl3): δ 21.42 (Me), 26.34 (CMe2), 40.07, 40.73 (2NMe2), 57.22 (HN-CH), 77.05 (HC-C-C), 102.26, (CMe2), 126.00 (2CH), 129.38 (2CH), 136.56 (C), 140.57 (C), 161.70 (N-C-NH), 166.65 (2C, s, 2C=O) ppm. MS, m/z (%): 361 (M+, < 1), 246 (91), 188 (84), 173 (97), 160 (30), 144 (22), 115 (100), 105 (16), 71 (45), 57 (18), 43 (34). Anal. Calcd for C19H27N3O4 (361.44): C, 63.14; H, 7.53; N, 11.63 Found: C, 63.27; H, 7.59; N, 11.58.
5-{((bis (dimethylamino) methylene) ammonio) (o-tolyl) methyl}-2, 2-dimethyl-4, 6-dioxo-1, 3-dioxin-5-ide (4c)
White powder, mp 187-189 °C (decomp), 0.300 g, (83 %). IR (KBr) (νmax/cm−1): 1635 (C=O), 1682 (C=N), 3229 (NH). 1H NMR (300.1 MHz, CDCl3): δ 1.53 (6H, s, CMe2), 2.38 (3H, s, Me), 2.90 and 2.95 (12H, 2s, 2NMe2), 5.85 (1H, d, 3JHH = 8.6 Hz, HN-CH), 7.20 (1H, t, 3JHH = 7.4 Hz, CH), 7.28 (2H, d, 3JHH = 7.4 Hz, 2CH), 7.56 (2H, d, 3JHH = 7.4 Hz, 2CH), 8.44. (1H, d, 3JHH = 8.6 Hz, NH) ppm. 13C NMR (75.4 MHz, CDCl3): δ 20.13 (Me), 26.23 (CMe2), 39.97, 40.44 (2NMe2), 55.29 (HN-CH), 76.47 (HC-C-C) 101.96 (CMe2), 126.09 (CH), 126.45 (CH), 126.91 (CH), 130.62 (CH), 134.96 (C), 140.77 (C), 161.45 (N-C-NH), 166.57 (2C, s, 2C=O) ppm. MS, m/z (%): 361 (M+, < 1), 246 (73), 188 (87), 173 (94), 160 (37), 144 (19), 115 (100), 105 (9), 71 (37), 57 (23), 43 (49). Anal. Calcd for C19H27N3O4 (361.44): C, 63.14; H, 7.53; N, 11.63. Found: C, 63.24; H, 7.59; N, 11.56.
5-{((bis (dimethylamino) methylene) ammonio) (m-tolyl) methyl}-2, 2-dimethyl-4, 6-dioxo-1, 3-dioxin-5-ide (4d)
White powder, mp 180-183 °C (decomp), 0.286 g, (79 %). IR (KBr) (νmax/cm−1): 1612 (C=O), 1682 (C=N), 3262 (NH). 1H NMR (300.1 MHz, CDCl3): δ 1.56 (6H, s, CMe2), 2.30 (3H, s, Me), 2.92 and 2.99 (12H, 2s, 2NMe2), 5.67 (1H, d, 3JHH = 8.6 Hz, HN-CH), 6.98-7.30 (4H, m,Ar), 9.00 (1H, d, 3JHH = 8.6 Hz, NH) ppm. 13C NMR (75.4 MHz, CDCl3): δ 21.93 (Me), 26.33 (CMe2), 40.08, 40.79 (2NMe2), 57.37 (HN-CH), 77.05 (HC-C-C) 102.32 (CMe2), 123.13 (CH), 126.70 (CH), 127.85 (CH), 128.60 (CH), 138.20 (C), 143.41 (C), 161.74 (N-C-NH), 166.66 (2C, s, 2C=O) ppm. MS, m/z (%):361 (M+, < 1), 246 (68), 188 (73), 173 (100), 160 (29), 115 (96), 105 (22), 71 (40), 57 (16), 43 (31). Anal. Calcd for C19H27N3O4 (361.44): C, 63.14; H, 7.53; N, 11.63. Found: C, 63.09; H, 7.47; N, 11.68.
5-{((bis (dimethylamino) methylene) ammonio) (4-chlorophenyl) methyl}-2, 2-dimethyl-4, 6-dioxo-1, 3-dioxin-5-ide (4e)
White powder, mp 188-190 °C (decomp), 0.267 g, (70 %). IR (KBr) (νmax/cm−1): 1612 (C=O), 1682 (C=N), 3186 (NH). 1H NMR (300.1 MHz, CDCl3): δ 1.52 (6H, s, CMe2), 2.87 and 2.93 (12H, 2s, 2NMe2), 5.61 (1H, d, 3JHH = 8.7 Hz, HN-CH), 7.19. (2H, d,J = 8.4 Hz, 2CH), 7.34 (2H, d,J = 8.4 Hz, 2CH), 8.83 (1H, d, 3JHH = 8.7 Hz, NH) ppm. 13C NMR (75.4 MHz, CDCl3): δ 26.33 (CMe2), 40.02, 40.74 (2NMe2), 56.82 (HN-CH), 77.11 (HC-C-C), 102.29 (CMe2), 127.53 (2CH), 128.71 (2CH), 132.57 (C), 142.08 (C), 161.83 (N-C-NH), 166.46 (2C, s, 2C=O) ppm. MS, m/z (%): 383 (M+ + 2, 1), 382 (M+ + 1, 1), 381 (M+, 4), 368 (20), 245 (91), 208 (58), 196 (39), 180 (46), 173 (100), 164 (48), 136 (74), 115 (30), 101 (30), 71 (55), 57 (31), 43 (37). Anal. Calcd for C18H24ClN3O4 (381.84): C, 56.62; H, 6.34; N, 11.00. Found: C, 56.52; H, 6.44; N, 11.12.
5-{((bis (dimethylamino) methylene) ammonio) (2-chlorophenyl) methyl}-2, 2-dimethyl-4, 6-dioxo-1, 3-dioxin-5-ide (4f)
White powder, mp 181-183 °C (decomp), 0.275 g, (72 %). IR (KBr) (νmax/cm−1): 1608 (C=O), 1679 (C=N), 3236 (NH). 1H NMR (300.1 MHz, CDCl3): δ 1.57 (6H, s, CMe2), 2.98 and 3.03 (12H, 2s, 2NMe2), 5.68 (1H, d, 3JHH = 8.7 Hz, HN-CH), 7.24-7.49 (4H, m,Ar), 9.00. (1H, d, 3JHH = 8.7 Hz, NH) ppm. 13C NMR (75.4 MHz, CDCl3): δ 26.37 (CMe2), 40.12, 40.72 (2NMe2), 56.76 (HN-CH), 77.09 (HC-C-C), 102.25, (CMe2), 125.38 (CH), 125.86 (CH), 127.34 (CH), 129.56 (CH), 134.23 (C), 146.07 (C), 161.95 (N-C-NH), 165.56 (2C, s, 2C=O) ppm. MS, m/z (%): 383 (M+ + 2, < 1), 382 (M+ + 1, < 1), 381 (M+, 2), 245 (84), 208 (43), 196 (27), 180 (27), 173 (100), 164 (33), 136 (80), 115 (53), 71 (39), 57 (24), 43 (39). Anal. Calcd for C18H24ClN3O4 (381.84): C, 56.62; H, 6.34; N, 11.00. Found: C, 56.68; H, 6.465; N, 11.10.
5-{((bis (dimethylamino) methylene) ammonio) (4-fluorophenyl) methyl}-2, 2-dimethyl-4, 6-dioxo-1, 3-dioxin-5-ide (4g)
White powder, mp 173-175 °C (decomp), 0.267 g, (73 %). IR (KBr) (νmax/cm−1): 1597 (C=O), 1682 (C=N), 3171 (NH). 1H NMR (300.1 MHz, CDCl3): δ 1.56 (6H, s, CMe2), 2.93 and 3.00 (12H, 2s, 2NMe2), 5.68 (1H, d, 3JHH = 8.6 Hz, HN-CH), 6.96 (2H, t,J = 8.7 Hz, 2CH), 7.49 (2H, dd,J = 8.7, 5.4 Hz, 2CH), 8.96 (1H, d, 3JHH = 8.6 Hz, NH) ppm. 13C NMR (75.4 MHz, CDCl3): δ 26.32 (CMe2), 40.12, 40.80 (2NMe2), 56.88 (HN-CH), 77.03 (HC-C-C), 102.37, (CMe2), 115.44 (d, 3JCF = 21 Hz, 2CH,), 127.69 (d, 2JCF = 7.5 Hz, 2CH), 139.38 (d, 4JCF = 3.1Hz, C), 161.79 (N-C-NH), 162.00 (d, 1JCF = 245 Hz, CF), 166.56 (2C, s, 2C=O) ppm. MS, m/z (%): 365 (M+, < 1), 245 (71), 192 (34), 173 (94), 160 (17), 148 (57), 115 (100), 71 (41), 57 (33), 43 (57). Anal. Calcd for C18H24FN3O4 (365.40): C, 59.17; H, 6.62; N, 11.50 Found: C, 59.04; H, 6.54; N, 11.58.
5-{((bis (dimethylamino) methylene) ammonio) (2-fluorophenyl) methyl}-2, 2-dimethyl-4, 6-dioxo-1, 3-dioxin-5-ide (4h)
White powder, mp 176-178 °C (decomp), 0.296 g, (81 %). IR (KBr) (νmax/cm−1): 1620 (C=O), 1678 (C=N), 3198 (NH). 1H NMR (300.1 MHz, CDCl3): δ 1.68 (6H, s, CMe2), 2.89 and 3.06 (12H, 2s, 2NMe2), 5.99 (1H, d, 3JHH = 9.4 Hz, HN-CH), 6.92-7.64 (4H, m,Ar), 8.28. (1H, d, 3JHH = 9.4 Hz, NH) ppm. 13C NMR (75.4 MHz, CDCl3): δ 26.49 (CMe2), 39.82, 40.45 (2NMe2), 51.38 (HN-CH), 72.87 (HC-C-C) 102.41 (CMe2), 115.37 (CH, d, 3 JFC = 21.3 Hz,), 125.2 (d, 2JFC = 9.4 Hz, CH), 129.6 (d, 3JFC = 22.4 Hz, CH), 129.8 (d, 2JFC = 9.9 Hz, C), 129.8 (CH, d, 4JFC = 3.0 Hz,), 160.83 (d, 1JFC = 243.2 Hz, CF) 161.01 (N-C-NH), 167.02 (2C, s, 2C=O) ppm. MS, m/z (%): 365 (M+, < 1), 245 (71), 173 (94), 160 (17), 148 (57), 115 (100), 71 (41), 57 (53), 43 (57). Anal. Calcd for C18H24FN3O4 (365.40): C, 59.17; H, 6.62; N, 11.50. Found: C, 59.06; H, 6.54; N, 11.57.
5-{((bis (dimethylamino) methylene) ammonio) (4-nitrophenyl) methyl}-2, 2-dimethyl-4, 6-dioxo-1, 3-dioxin-5-ide (4i)
Yellow powder, mp 191-193 °C (decomp), 0.302 g, (77 %). IR (KBr) (νmax/cm−1): 1612 (C=O), 1682 (C=N), 3194 (NH). 1H NMR (300.1 MHz, CDCl3): δ 1.55 (6H, s, CMe2), 2.92 and 3.01 (12H, 2s, 2NMe2), 5.73 (1H, d, 3JHH = 8.9 Hz, HN-CH), 7.71. (2H, d,J = 8.7 Hz, 2CH), 8.13 (2H, d,J = 8.7 Hz, 2CH), 8.92 (1H, d, 3JHH = 8.9 Hz, NH) ppm. 13C NMR (75.4 MHz, CDCl3): δ 26.34 (CMe2), 40.28, 40.89 (2 NMe2), 57.11 (HN-CH), 77.07 (HC-C-C), 102.61, (CMe2), 124.03(2CH), 126.78 (2CH), 147.01 (C), 150.69 (C), 162.12 (N-C–NH), 166.41 (2C, s, 2C=O) ppm. MS, m/z (%): 392 (M+, < 1), 245 (100), 219 (24), 196 (92), 152 (52), 122 (5), 115 (4), 71 (22), 57 (20), 43(18). Anal. Calcd for C18H24N4O6 (392.41): C, 55.09; H, 6.16; N, 14.28. Found: C, 55.22; H, 6.19; N, 14.16.
5-{((bis (dimethylamino) methylene) ammonio) (4-methoxyphenyl) methyl}-2, 2-dimethyl-4, 6-dioxo-1, 3-dioxin-5-ide (4j).
White powder, mp 169-171 °C (decomp), 0.321 g, (85 %). IR (KBr) (νmax/cm−1): 1612 (C=O), 1682 (C=N), 3194 (NH). 1H NMR (300.1 MHz, CDCl3): δ 1.55 (6H, s, CMe2), 2.90 and 2.96 (12H, 2s, 2NMe2), 3.74 (3H, s, OMe), 5.63 (1H, d, 3JHH = 8.4 Hz, HN-CH), 6.78 (2H, d,J = 8.7 Hz, 2CH), , 7.39 (2H, d,J = 8.7 Hz, 2CH), 8.94 (1H, d, 3JHH = 8.4 Hz, NH) ppm. 13C NMR (75.4 MHz, CDCl3): δ 26.34 (CMe2), 40.05, 40.71 (2NMe2), 55.62 (OMe), 56.93 (HN-CH), 77.08 (HC-C-C), 102.24, (CMe2), 114.05 (2CH), 127.29 (2CH), 135.91 (C), 158.71 (C), 161.62 (N-C-NH), 166.62 (2C, s, 2C=O) ppm. MS, m/z (%): 377 (M+, 1), 246 (82), 204 (19), 189 (11), 173 (100), 160 (62), 121 (19), 115 (57), 71 (34), 57 (43), 43 (31). Anal. Calcd for C19H27N3O5 (377.43): C, 60.46; H, 7.21; N, 11.13. Found: C, 60.54; H, 7.27; N, 11.06.
5-{((bis (dimethylamino) methylene) ammonio) (4-(dimethylamino) phenyl) methyl}-2, 2-dimethyl-4, 6-dioxo-1, 3-dioxin-5-ide (4k)
White powder, mp 123-125 °C (decomp), 0.289 g, (74 %). IR (KBr) (νmax/cm−1): 1604 (C=O), 1682 (C=N), 3333 (NH). 1H NMR (300.1 MHz, CDCl3): δ 1.77 (6H, s, CMe2), 2.96 and 2.99 (12H, 2s, 2NMe2), 3.16 (Ar-NMe2), 5.66 (1H, d, 3JHH = 7.8 Hz, HN-CH), 6.68 (2H, d,J = 8.7 Hz, 2CH), 8.27 (2H, d,J = 8.7 Hz, 2CH), 8.93 (1H, d, 3JHH = 7.8 Hz, NH) ppm. 13C NMR (75.4 MHz, CDCl3): δ 26.34 (CMe2), 39.81, 40.48 (2NMe2), 41.05 (Ar-NMe2) 56.90 (HN-CH), 77.01 (HC-C-C), 102.19, (CMe2), 112.92 (2CH), 127.06 (2CH), 131.69 (C), 149.88 (C), 161.52 (N-C-NH), 166.78 (2C, s, 2C=O) ppm. MS, m/z (%): 390 (M+, < 1), 246 (89), 217 (21), 173 (94), 160 (31), 142 (16), 115 (100), 89 (27), 71 (42), 57 (24), 43 (42). Anal. Calcd for C20H30N4O4 (390.23): C, 61.52; H, 7.74; N, 14.35. Found: C, 61.60; H, 7.84; N, 14.39.
5-{((bis (dimethylamino) methylene) ammonio) (4-bromophenyl) methyl}-2, 2-dimethyl-4, 6-dioxo-1, 3-dioxin-5-ide (4l)
White powder, mp 204-206 °C (decomp), 0.324 g, (76 %). IR (KBr) (νmax/cm−1): 1613 (C=O), 1682 (C=N), 3193 (NH). 1H NMR (300.1 MHz, CDCl3): δ 1.574 (6H, s, CMe2), 2.89 and 2.96 (12H, 2s, 2NMe2), 5.62 (1H, d, 3JHH = 8.7 Hz, HN-CH), 7.38 (4H, s, Ar) 8.87 (1H, d, 3JHH = 8.7 Hz, NH) ppm. 13C NMR (75.4 MHz, CDCl3): δ 26.35 (CMe2), 40.05, 40.81 (2NMe2), 56.91 (HN-CH), 77.07 (HC-C-C), 102.36, (CMe2), 120.73 (C), 127.91 (2CH), 131.69 (2CH), 142.57 (C), 161.87 (N-C-NH), 166.50 (2C, s, 2C=O) ppm. MS, m/z (%): 427 (M+, < 1), 425 (M+, < 1), 325 (14), 246 (58), 253 (21), 173 (89), 209 (19), 160 (31), 115 (100), 71 (29), 57 (24), 43 (38). Anal. Calcd for C18H24BrN3O4 (426.30): C, 61.52; H, 7.74; N, 14.35. Found: C, 61.60; H, 7.81; N, 14.39.
Results and Discussion
The three component condensation reactions of Meldrum acid 1, benzaldehyde derivatives 2, and N, N, N’, N’-Tetramethyl-guanidine 3 proceeded with piperidinium acetate at room temperature in benzene and were complete within about a day. Analysis of the IR, 1H and 13C NMR spectroscopy, and mass spectrometry spectra as well as elemental analysis confirm the formation and isolation of the desired zwitterion salts as shown in scheme 1. The IR spectrum of 4b exhibits a NH stretching band at 3236 cm-1, signals for carbonyl groups at 1601 cm-1 and for C=N at 1682 cm-1. The 1H NMR spectrum of 4b exhibited four single sharp lines readily recognized as CMe2 (δ = 1.55 ppm), Ar-Me (δ = 2.28 ppm), two NMe2 groups (δ = 2.92, 2.98 ppm), along with two characteristic doublets (δ = 5.67 and 8.97 ppm, 3JHH = 8.5) for the H-C-NH moiety. All aromatic protons resonate at δ = 7.05-7.38 ppm.
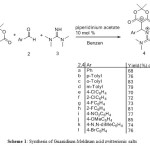 |
Scheme1: Synthesis of Guanidium-Meldrum acid zwitterionic salts
|
The 1H-decoupled 13C NMR spectrum of 4b showed six distinct signals below 100 ppm, which were readily recognized as arising from Ar-Me (δ = 21.42 ppm), CMe2 groups (δ = 26.34 ppm), two NMe2 groups (δ = 40.06, 40.73 ppm), methine (δ = 57.22, 77.05 ppm) carbon atoms. The meldrum acid residue showed only one signal for the carbonyl (δ = 166.65) group, which is consistent with the presence of a local Cs symmetry, which exhibited characteristic signals with appropriate chemical shifts. The 1H and 13C NMR spectra of the other compounds are similar to those of 4b except for the aryl groups, which exhibit characteristic signals with appropriate chemical shifts. The mass and elemental analyses data were in agreement with the proposed structures. The formation of zwitterionic salts 4 can be rationalized by initial formation of a conjugated electron-deficient heterodyne 5 through Knoevenagel condensation (16) of the 1 and benzaldehydes 2 using piperidinium acetate followed by a Michael-type 9, 11 addition reaction with N, N, N’, N’-Tetramethyl-guanidine 3 to afford compound 4 as shown in scheme 2. We didn’t show Cyclization of synthetic compounds at room temperature, 12 refluxing for 5h and microwave irradiation.
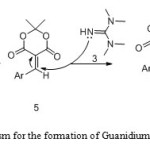 |
Scheme2: Proposed mechanism for the formation of Guanidium-Meldrum acid zwitterionic salts Click here to View scheme |
The 1H NMR spectrum of 4b in CDCl3 at ambient temperature displayed two single resonances due to the NMe2 (δ = 2.96, 3.02) protons. At about + 30 °C, the resonances arising from the NMe2 protons were appreciably broadened when compared to the corresponding signals at room temperature, whereas other group’s resonances remained unchanged. The NMe2 protons coalescences near + 35 °C and appeared as a fairly symmetrical line at + 40 °C. The variable temperature spectra allowed calculating the free-energy barrier for the C-NH bond two NMe2 groups rotation in 4b (Scheme 3). Using the expression k = πΔυ/√2, first order rate constant (k = 39.96 s-1) was calculated for the N-C bond rotation in 4b at 308 K as shown in Table. Application of the absolute rate theory with a transmission coefficient of 4b gave free-energy activation (ΔG#) of 66 ± 2 k Jmol-1, where all known sources of errors were estimated and included. 14 Only two 1:1 singlets are observed for the four diastereotopic methyl groups of the guanidino moiety in the 1H and 13C NMR spectra of 4b at room temperature. Two rotational processes, i) C–NH bond rotation (A, B) and ii) C–NMe2 bond rotation (C), can be envisaged in the guanidino moiety of 4. When both of these processes are fast on the NMR timescale, the four Me groups become equivalent and exhibit a single resonance, as for the spectra observed above 308 K. two observed signals for these groups’ shows one of the processes should be fast on the NMR timescale. We conceder the contribution of the valence-bond structures A and B to the resonance hybrid of 4 is in fact more important than that of C for the simple reason that they correspond to a more highly substituted carbon–nitrogen double bond. 14 Therefore the faster process can be the C–NH rotation (process i). In summary, we have reported the synthesis of guanidinium zwitterionic salts by an efficient and simple approach along with three component condensation in benzene in presence of piperidinium acetate at room temperature within one day. A dynamic NMR effect is observed in the 1H NMR spectra of these compounds as a result of restricted rotation around C-NH bond for two NMe2 groups.
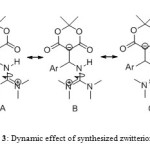 |
Scheme3: Dynamic effect of synthesized zwitterionic salts Click here to View scheme |
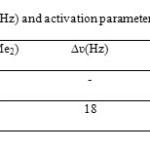 |
Table: Selected 1H chemical shifts (300 MHz) and activation parameters of 4b in CDCl3 Click here to View table |
Acknowledgment
Author gratefully acknowledges financial support from the Research Council of Khoy Branch Islamic Azad University.
References
- a: McNab, H. Chem. Soc. Rev., 1978, 7, 345-358. b: Chen, B. C. Heterocycles, 1991, 32, 529-597. c: Dumas. A. N.; Fillion, E. Acc. Chem. Res., 2010, 43, 440-454.
- a: Ayoubi, S. A.; Texier-Boullet, F.; Hamelin, J. Synthesis, 1994, 258-260. b: Ayoubi, S. A.; Texier-Boullet, F. J. Chem. Res. (S), 1995, 205-206. c: Jones, G. Organic Reactions, vol. 15, Wiley, New York, 1967, p 204. d: Choudary, B. M.; Kantam, M. L.; Neeraja, V.; Rao, K. K.; Figueras, F.; Delmotte, L. Green Chem., 2001, 3, 257-260. e: Narsaiha, A. V.; Basak, A. K.; Visali, B.; Nagaiah, K. Synth Commun., 2004, 34, 2893-2901. f: Desai, U. V.; Pore, D. M.; Mane, R. B.; Solabanavar, S. B.; Wadgaonkar, P. P. Synth. Commun. 2004, 34 (1), 25-32. g: Fildes, D.; Caignaert, V.; Villemin, D.; Jaffres, P. Green Chem. 2001, 3, 52-56. h: Rao, P. S.; Venkataratnam, R. V. Indian J. Chem. B, 1993, 32, 484-486. i: Dumas, A. M.; Seed, A.; Zorzitto, A. K.; Fillion, E. Tetrahedron Letters, 2007, 48, 7072-7074. j: Kraus G. A.; Krolski, M. E. J. Org. Chem. 1986, 51, 3347-3350. k: Thorat, M. T.; Jagdale, M. H.; Mane, R. B.; Salunkhe, M. M.; Wadgaonkar, P. P. Curr. Sci, 1987, 56 (15), 771-772. l: Bigi, F.; Carloni, S.; Ferrari, L.; Maggi, R.; Mazzacani, A.; Sartori, G. Tetrahedron Lett. 2001, 42, 5203-5205. m:. Hedge, A.; Kruse, C. W.; Snyder, H. R. J. Org. Chem. 1961, 26, 3166-3170. n: Darvatkar, N. B.; Deorukhkar, A. R.; Bhilare, S. V.; Salunkhe, M. M. Synthetic Communications, 2006, 36, 3043-3057.
- a: Wilsily, A.; Fillion, E. J. Org. Chem. 2009, 74, 8583-8594. b: Knopfel, T. F.; Zarotti, P.; Ichikawa, T.; E. Carreira, M. J Am. Chem. Soc. 2005, 127, 9682-9683. c: Ziegler, F. E.; Guenther, T.; Nelson, R. V. Synth. Commun. 1980, 10, 661-665.
- a: Patasz, A.; Jelska, K.; Ozóg, M.; Serda, P. Monatsh. Chem. 2007, 138, 481-488. b: Brown, R. C. F.; McMullen, G. L. Aust. J. Chem. 1974, 27, 2385-2391.
- Mahulikar, P. P.; Mane, R. B. J. Chem. Res. 2006, 4, 15-18.
- Baxter, G. J.; Brown, R. F. C.; McMullen, G. L. Aust. J. Chem. 1974, 27, 2605-2610.
- Brown, R. C. F.; Eastwood, F. W., Harrington, K. J. Aust. J. Chem. 1974, 27, 2373-2384.
- Fan, M. J.; Qian, B.; Zhao, L. B.; Liang, Y. M. Tetrahedron, 2007, 63, 8987-8992.
- Esmaeili, A. A.; Zendegani, H. Tetrahedron, 2005, 61, 4031-4034.
- Nair, V.; Unni Vinod, A.; Abhilash, N.; Menon, R. S.; Santhi, V.; Luxmi Varma, R.; Viji, S.; Mathewa, S.; Srinivas, R. Tetrahedron, 2003, 59, 10279-10286.
- Yavari, I.; Aminkhani A.; Arab-Salmanabadi, S. Monatsh. Chem. 2012, 143, 1195-1198.
- Yavaria; I.; Shahvelayatia; A. Malekafzalia, A. Journal of Sulfur Chemistry 2010, 31(6), 499–508.
- Oki, M.; Application of Dynamic NMR Spectroscopy to Organic Chemistry, VCH publishers, Deerfield. Beach, Florida, 1985.
- Yavari, I. Roberts, J. D.; Biochem Biophys Commun, 1978, 83, 635-640.

This work is licensed under a Creative Commons Attribution 4.0 International License.









