Fe3O4 nanoparticles modified with APTES as the carrier for (+)-(S)-2-(6-methoxynaphthalen-2-yl) propanoic acid (Naproxen) and (RS) 2-(3-benzoylphenyl)-propionic acid (Ketoprofen) drug
Farzaneh Hosseini1, Mirabdullah Seyed Sadjadi 1*, Nazanin Farhadyar2
1Department of Chemistry, Science and Research Branch, Islamic Azad University, Tehran, Iran
2Department of Chemistry, Varamin-Pishva Branch, Islamic Azad University, Tehran, Iran
DOI : http://dx.doi.org/10.13005/ojc/300420
Article Received on :
Article Accepted on :
Article Published : 09 Dec 2014
Modified Fe3O4 nanoparticles with (3-aminopropyl) triethoxysilane (APTES) were synthesized by post grafting method for loading the anti-inflammatory drug: (+)-(S)-2-(6-methoxynaphthalen-2-yl) propanoic acid –Naproxen and (RS) 2-(3-benzoylphenyl)-propionic acid -Ketoprofen. The prepared samples were characterized by X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), Field Emission Scanning Electron Microscopy (FE-SEM), Energy Dispersive X-Ray Spectroscopy (EDX), Vibrating sample magnetometer (VSM), and Dynamic light scattering (DLS) diagrams. These nanoparticles have surface with free - NH2 groups can carry out ionic interaction with carboxylic groups and act as a carrier of drugs.
KEYWORDS:Nanoparticle; Carrier; Drug delivery; Naproxen; Ketoprofen
Download this article as:| Copy the following to cite this article: Hosseini F, Seyedsadjadi M, Farhadyar N. Fe3O4 nanoparticles modified with APTES as the carrier for (+)-(S)-2-(6-methoxynaphthalen-2-yl) propanoic acid (Naproxen) and (RS) 2-(3-benzoylphenyl)-propionic acid (Ketoprofen) drug. Orient J Chem 2014;30(4). |
| Copy the following to cite this URL: Hosseini F, Seyedsadjadi M, Farhadyar N. Fe3O4 nanoparticles modified with APTES as the carrier for (+)-(S)-2-(6-methoxynaphthalen-2-yl) propanoic acid (Naproxen) and (RS) 2-(3-benzoylphenyl)-propionic acid (Ketoprofen) drug. Available from: http://www.orientjchem.org/?p=5636 |
Introduction
In the past decade , targeted drug delivery technology has been enormous attention in medicine and pharmaceutical industries due to much advantage compared to conventional such as low toxicity , biocompatibility [1], improving existing drugs’, therapeutic efficacy, alleviating their side effects, reducing the cost and so on[2]. Nanotechnology has firmly entered the domain of drug delivery. Different Nano carriers including dendrimers [3], micelles [4], emulsions [5] liposomes [6] and magnatic nanoparticles [7-10] and etc, are used to target specific areas in the body. Because of the unique characteristics of magnetic nanoparticles such as superparamagnetism, high coercivity, low Curie temperature, and high magnetic susceptibility have gained much scientific interest [11-12]. Drug-carrying magnetic nanoparticles can be concentrated in cancer tissue by external magnetic fields [13]. Internalization of magnetic nanoparticles strongly depends upon the particle size. These applications require the magnetic nanoparticles in size smaller than 100 nm and a narrow particle size distribution. Larger particles with a diameter higher than 200 nm are easily isolated by the spleen and finally eliminated by the cells of the phagocyte system, thus it leads to a reduction of blood circulation times. Small particles with diameters less than 10 nm are rapidly removed through extravasations and renal clearance. Particles with a diameter ranging from 10 to 100 nm might be considered optimal for intravenous injection and have the most prolonged blood circulation time. These particles are small enough to evade the RES of the body as well as to penetrate small capillaries of the tissues and offer the most effective distribution in targeted tissues. [14]. When the magnetic nanoparticles is used uncoated as drug carriers, they have lower performance because of some limitations in drug loading, retention time ,and release rates in the blood stream [15,16]. Coated magnetic nanoparticles with silica, gold, or polymers [14] not only overcome these problems but also to avoid the formation of aggregates and provide functional groups (amines or carboxylic acid) for help in binding various biological ligands [17]. Types of polymeric surface coatings(organic and inorganic) have been used such as dextran, carboxymethylated dextran, carboxydextran, starch, arabinogalactan, glycosaminoglycan, sulfonated styrene-divinylbenzene, polyethylene glycol (PEG), polyvinyl alcohol (PVA), poloxamers, polyoxamines [14], Polyvinylpyrrolidone-iodine [18] and chitosan [19]. The natural polymers are more important because these materials are more biocompatibility [14]. Silica shells are appropriate options to be employed as protective coatings on iron oxide nanoparticles thanks to their stability under aqueous conditions and ease of synthesis [20]. Trialkoxysilanes , bifunctional molecules ,entail a trialkoxy group that they are granted to modify the surface of nanoparticles . (3-Aminopropyl) triethoxysilane is intended to be done through the grafting of aminopropylsilane groups (–O)3 Si–(CH2)3–NH2 via formation of covalent bonds which are bound to the particle surface and makes basic surface. Following prior step, it would be regarded as nanocarrier attracting acidic drugs resulted in an ionic interaction [21, 22]. Modified magnetic nanoparticles have been synthesized by two methods. In the first method, nanoparticles are coated during the synthesis that is in situ coatings. [23]. The post-synthesis coating method consists of grafting the polymer on the magnetic particles once synthesized [24-26] (polymeric surfactants). This paper provides a detailed study of the preparation iron oxide nanoparticles modified with APTES by post grafting method and the anti-inflammatory drug: (+)-(S)-2-(6-methoxynaphthalen-2-yl) propanoic acid –Naproxen and (RS) 2-(3-benzoylphenyl)-propionic acid –Ketoprofen were loaded onto them. The morphology/size and magnetization was determined for these nanoparticles using Field Emission Scanning Microscopy (FE-SEM), X-ray powder diffraction and VSM respectively. Fourier transform infrared spectroscopy was employed in order to identify the presence of APTES, ketoprofen and Naproxen drugs on Fe3o4 nanoparticles surface. Hydrodynamic size of ketoprofen-APTES-nanoparticles was designated by Dynamic light scattering (DLS).
Materials and Methods
Reagents and Materials
Ferric chloride hexahydrate (FeCl3, 6H2O), (3-aminopropyl) triethoxysilane (APTES), (+)-(S)-2-(6-methoxynaphthalen-2-yl) propanoic acid (Naproxen), and (RS) 2-(3-benzoylphenyl)-propionic acid (Ketoprofen) were obtained from Sigma-Aldrich. Iron (III) sulfate heptahydrate (FeSO4, 7H2O) and ammonium hydroxide 25 wt% were purchased Fluka (Buchs, Switzerland).
Synthesis of Fe3O4 Nanoparticles
Iron oxide magnetic nanoparticles were prepared by a conventional co-precipitation. In summary, Sodium hydroxide solutions (250 mL, 1M) were added to a three-neck round-bottomed flask under protection of argon flow. The solution was heated to 85°C. Then 12 ml of deionized water containing 4.04 g of Iron(III) nitrate and 1.39 g of Iron(III) sulfate (FeSO4,7H2O) were added dropwise, while stirring vigorously until a black precipitate was formed. The mixture was kept at this condition for 1 h. To remove the remaining ions, the generated precipitate was centrifuged and washed at least three times until a pH value of 7 was achieved. The powder was dried at 60°C for 24 hours.
Modification of Fe3O4 Nanoparticles By (3-Aminopropyl) Triethoxysilane
The obtained magnetite nanoparticles powder (1 g) was dispersed in 150 mL ethanol/water (volume ratio, 1:1) solution by sonication for 30 min. After that, (3-aminopropyl) triethoxysilane (APTES) (99%, 3 mL) were added to the mixture.
Then resulting mixture was stirred under argon atmosphere and 400c for 24 hours. The final product was separated from the solution and washed for 5 times by water, acetone and ethanol. The precipitated product (APTES– Fe3O4) was dried at room temperature under vacuum.
Adsorption of Naproxen and Ketoprofen Drugs on APTES- Fe3O4 Nano Carrier’s Surface
2.0 g of APTES- Fe3O4 introduced to 100 mL of 2-propanol solution containing drug (10 mg/ mL). The adsorption was carried out at room temperature for 24 h. After magnetic separation, drug nanocarriers were perfectly washed by 2-propanol solution and dried at room temperature (24 h). Figure 1 indicates a schematic of these approaches.
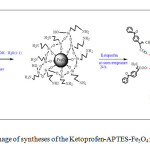 |
Fig1: schematic image of syntheses of the Ketoprofen-APTES-Fe3O4 nanoparticles Click here to View figure |
Results and Discussion
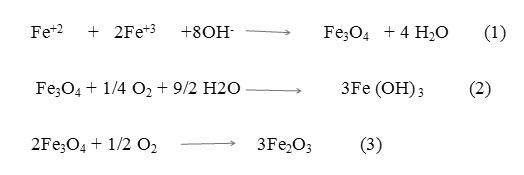
Preparing of iron oxide nanoparticles was carried out by the co precipitation method in an aqueous medium, through reaction (1). If the nanoparticles are exposed in the presence of oxygen or air, might undergo oxidation to Fe(OH)3 or as shown in reaction (2) [27], or to Fe2O3 phase according to reaction (3) [28]. So the reaction was carried out under nitrogen gas continuously.
Iron oxide nanoparticles surface modified by the process Silanization. This reaction involves the covering of a surface iron oxide nanoparticles through self-assembly with (3-aminopropyl)-triethoxysilane molecules. During this reaction ,hydroxyl groups on the surface of iron oxide nanoparticles attack and replace ethoxy groups of APTES ,thus is formed a covalent -Si-O-Si- bond and amino propyl-terminated surface((see Figure1). The surface coating of nanoparticles by APTES depends on experimental parameters such as reaction time, temperature and silane concentration. Interaction of ketoprofen and Naproxen drugs (carboxylic acid) with basic amino propyl-terminated surface of iron oxide nanoparticles is an ionic interaction. Rosenholm and Lindén [29] show that in polar solvents like 2-propanol used in this research was possible.
Characterization of the Samples
X-ray Powder Diffraction
Fig. 2 shows the results of X-ray diffraction analysis for naked Fe3o4 and APTES @Fe3O4 nanoparticles. This figure indicates that the predominant phase of constituted iron oxide is Fe3o4 (magnetite). Because the position and relative intensities of all peaks in XRD obtained patterns are in good agreement with the standard diffraction spectrum (JCPDS Card No. 19-0629) [30] and Peaks of Fe(OH)3 (d= 3.376 at 2θ= 26.380 ), goethite (d =4.183 Ao at 2 θ =21.220), hematite (d=2.700 Ao at 2 =33.150 ) [28] were not observed. A weak broad band (2θ = 17–26◦) can be seen in XRD pattern of APTES- Fe3O4 can be devoted to amorphous silane shell formed Surrounding magnetic core [31]. The average particle size was estimated by Sherrer’s equation: D = Kλ/ (βCosθ). Where D is equivalent of particles average core diameter; K is the grain shape factor (K=0.94); λ is X-ray wavelength (1.54060A0); β denotes the full width at half-maximum or FWHM (in radians) of the highest intensity 311 powder diffraction reflection, and θ is the Bragg angle. FWHM and 2θ values for naked Fe3O4 and APTES -Fe3O4 nanoparticles, are respectively included 1.38, 35.63Ao and 1.59, 35.73 Ao. , Considering these data, both naked Fe3O4 and APTES-Fe3O4 exhibited sizes approximately equal to 6 nm. Although thermal treatment can grow in size and modify nanoparticles physical properties but the same size observed for naked Fe3O4 and APTES -Fe3O4 nanoparticles, show that thermal treatment in during the silanization reaction was not enough to cause growth and accordingly dramatic effect on the physical properties of the iron oxide particles [28].
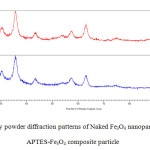 |
Fig2: X-ray powder diffraction patterns of Naked Fe3O4 nanoparticles and APTES-Fe3O4 composite particle Click here to View figure |
Fourier Transforms Infrared Spectra
Fig. 3 indicates the FTIR spectra of the naked Fe3O4 and APTES -Fe3O4 carrier before and after ketoprofen and naproxen drugs adsorption. The Sharp and revealing peak at around 580-594 cm-1 can be observed in (a), (b), (e), and (f) spectra is relates to the absorption peak Fe- O -Fe bond of Fe3O4 nanoparticles. This peak appears for bulk Fe3O4 at 570 and 575 cm−1.This blue shift is a result of decrease in the size of iron oxide [32, 33]. APTES presence on the surface of Fe3o4 nanoparticles is proven by the bands at 996 and 1126cm−1 that dedicated to the Si –O stretching vibrations and the broad band at 3401cm−1 that is assigned to the N–H stretching vibration (Fig 3b) [34]. The presence of the propyl group of APTES was confirmed by C–H stretching vibrations that appeared at 2862 cm-1. Adsorption Of ketoprofen and naproxen drugs on APTES-Fe3O4 nanoparticles resulted in disappearance of the absorption band at 1720 and 1728 cm−1 (Fig. 3c and d) characteristic to carbonyl stretching vibrations in carboxylic groups in adsorbed ketoprofen and naproxen drugs respectively and appearance of them characteristic bands at 1638 and 1630 cm-1 (Fig. 3g and h) related to stretching vibrations of ionized carboxylic groups were seen [35]. This observation confirms the ionic interaction and conjugtion between the drug and the APTES-Fe3O4. Moreover, in drug- conjugated Fe3o4 nanocomposite presence of many characteristic peaks of ketoprofen and naproxen drugs such as c=c stretching vibration peak of aromatic group at 1375,1430 and 1605,1462 cm-1(Fig. 3g and h) corroborate the conjugtion of drug to the APTES-Fe3O4 carrier. To compare the absorption peaks corresponding to Figure 3 are listed in Table 1. The part of FTIR spectrum show exhibiting absorption band of c=c stretching vibration of aromatic group of naproxen (g) and ketoprofen (h) loaded on APTES-Fe3O4 nanoparticles.
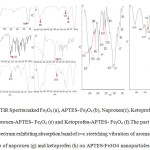 |
Fig3: FTIR Spectra naked Fe3O4 (a), APTES- Fe3O4 (b), Naproxen(c), Ketoprofen (d), Naproxen-APTES- Fe3O4 (e) and Ketoprofen-APTES- Fe3O4 (f).The part of FTIR spectrum exhibiting absorption band of c=c stretching vibration of aromatic group of naproxen (g) and ketoprofen (h) on APTES-Fe3O4 nanoparticles
|
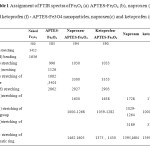 |
Table1: Assignment of FTIR spectra of Fe3O4 (a) APTES-Fe3O4 (b), naproxen (e) and ketoprofen (f) – APTES-Fe3O4 nanoparticles, naproxen(c) and ketoprofen (d) Click here to View table |
Field Emission Scanning Microscopy
The surface morphology of naked Fe3O4, APTES-Fe3O4, naproxen and ketoprofen – APTES-Fe3O4 nanoparticles, was observed by scanning electron microscopy. Fig. 4(a – d) shows the FE-SEM images of these nanoparticles respectively. As shown in from these images, the formation of nanoparticles is nearly uniform and spherical shape with homogeneously dispersed. In other words, during the silanization reaction and drug loading, morphological properties of nanoparticles do not noticeably change.
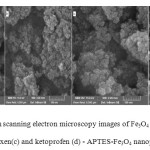 |
Fig4: Field emission scanning electron microscopy images of Fe3O4 (a), APTES Fe3O4 (b), naproxen(c) and ketoprofen (d) – APTES-Fe3O4 nanoparticles Click here to View figure |
Energy Dispersive X-ray Analysis (EDX)
The surface composition of naked Fe3O4, APTES-Fe3O4, naproxen and ketoprofen – APTES-Fe3O4 nanoparticles was designated by energy-dispersive X-ray spectroscopy as shown in Figure 5 and table 2. The presence of iron and oxygen can be seen in all of the samples, with iron abundance more than oxygen. APTES presence on the surface of Fe3O4 nanoparticles was proven by increase of percentage C and Si (b). Also Ketoprofen and naproxen drug adsorption on the surface of APTES-Fe3O4 nanoparticles is confirmed by the increase in carbon atomic and weight percent.
 |
Fig5: Edx result of naked Fe3O4 (a), APTES-Fe3O4 (b), naproxen(c) and ketoprofen (d) – APTES-Fe3O4 nanoparticles Click here to View figure |
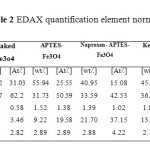 |
Table2: EDAX quantification element normalized Click here to View table |
Dynamic Light Scattering (DLS) Diagrams
The size histogram of naproxen (a) and ketoprofen (b) -APTES- Fe3O4 is shown in Fig. 6. Particles size was further identified by Zetasizer using DLS. These figures suggest that more than 50% of the atoms have hydrodynamic size below 100 nm. In drug delivery systems, the entry of nanoparticles to target tissue strongly relies on the size of the particles. Particles with a diameter ranging from 10 to 100 nm might be considered optimal for intravenous injection and have the most prolonged blood circulation time [14].
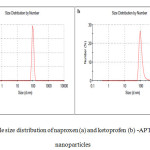 |
Fig6: particle size distribution of naproxen (a) and ketoprofen (b) -APTES- Fe3O4 nanoparticles Click here to View figure |
Vibrating scanning Magnetometry (VSM)
The magnetic properties of naked iron oxide and naproxen-APTES- iron oxide nanoparticles were characterized by vibrating sample magnetometry. VSM graphs of these samples are presented in Fig. 7. As it is obvious from this figure, naked iron oxide nanoparticles and drug-APTES- iron oxide nanoparticles have a hysteresis loop with zero coercivity and remanence values or super paramagnetic behaviors, super paramagnetism occurs when the particles sufficiently small so that thermal fluctuations can overcome the magnetic anisotropy. The saturation magnetization value of naked iron oxide and naproxen -APTES- iron oxide nanoparticles were found to be 55.4and 45.5 electromagnetic units per gram (emu/g) respectively. The reduction in saturation magnetization was likely due to the existence of APTES on surface of Fe3O4 nanoparticles.
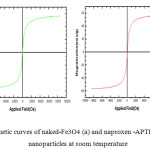 |
Fig7: Magnetic curves of naked-Fe3O4 (a) and naproxen -APTES-Fe3O4(b)- nanoparticles at room temperature Click here to View figure |
Conclusions
Iron oxide magnetic nanoparticles were prepared by a conventional co-precipitation and modified by (3-aminopropyl) triethoxysilane (APTES) .The modification of Fe3O4 nanoparticles leads to the formation of nanocarriers with surface basic properties. Two anti-inflammatory drug: (+)-(S)-2-(6-methoxynaphthalen-2-yl) propanoic acid –Naproxen and (RS) 2-(3-benzoylphenyl)-propionic acid –Ketoprofen were loaded on the surface of nanocarriers The adsorption of drugs is due to ionic interactions between the amine functional group of APTES and the carboxylic group of drugs, that confirmed by Fourier transform infrared spectra. The most part of nanocarriers loaded with drug has size less than 100 nm and due to inherent magnatic characteristic(45.5 emu/g ) they are able to penetrate the target tissue in attended of external magnetic fields.
References
- Muhammad Irfan Majeed, Qunwei Lu, Wei Yan, Zhen Li , Irshad Hussain, Muhammad Nawaz Tahir, Wolfgang Tremele and Bien Tan , Highly water-soluble magnetic iron oxide (Fe3O4) nanoparticles for drug delivery: enhanced in vitro therapeutic efficacy of doxorubicin and MION conjugates . J. Mater. Chem. B, 1)2013(2874–2884
- Li Yan, Xianfeng Chen ,Nanomaterials for Drug Delivery Nanocrystalline Materials (Second Edition) ( 2014) 221-268
- Ali Pourjavadi, Seyed Hassan Hosseini, Mahshid Alizadeh, Craig Bennett Magnetic pH-responsive nanocarrier with long spacer length and high colloidal stability for controlled delivery of doxorubicin Original Research Article Colloids and Surfaces B: Biointerfaces 116 ( 2014) 49-54
- Lin Zhu, Federico Perche, Tao Wang, Vladimir P. Torchilin Matrix metalloproteinase 2-sensitive multifunctional polymeric micelles for tumor-specific co-delivery of siRNA and hydrophobic drugsOriginal Research Article Biomaterials, Volume 35, Issue 13 ( 2014) 4213-4222.
- Noraini Ahmad, Roland Ramsch, Meritxell Llinàs, Conxita Solans, Rauzah Hashim, Hairul Anuar TajuddinInfluence of nonionic branched-chain alkyl glycosides on a model nano-emulsion for drug delivery systems Original Research Article Colloids and Surfaces B: Biointerfaces, Volume 115, (2014) 267-274.
- Kazuaki Ninomiya, Takahiro Yamashita, Shinya Kawabata, Nobuaki Shimizu, Targeted and ultrasound-triggered drug delivery using liposomes co-modified with cancer cell-targeting aptamers and a thermosensitive polymer Original Research Article Ultrasonics Sonochemistry, Volume 21, Issue 4, (2014) 1482-1488.
- Omid Veiseh, Jonathan W. Gunn, Miqin Zhang, Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging Review Article Advanced Drug Delivery Reviews, Volume 62, Issue 3(2010) 284-304
- Conroy Sun, Jerry S.H. Lee, Miqin Zhang, Magnetic nanoparticles in MR imaging and drug delivery Review Article Advanced Drug Delivery Reviews, 60, Issue 11, ( 2008) 1252-1265
- Magdalena Hałupka-Bryl, Kei Asai, Sindhu Thangavel, Magdalena Bednarowicz, Ryszard Krzyminiewski, Yukio Nagasaki, Synthesis and in vitro and in vivo evaluations of poly(ethylene glycol)-block-poly(4-vinylbenzylphosphonate) magnetic nanoparticles containing doxorubicin as a potential targeted drug delivery systemOriginal, Research Article Colloids and Surfaces B: Biointerfaces, In Press, Accepted Manuscript,Available online 8 April 2014
- Marcu, A.; Pop, S.; Dumitrache, F.; Mocanu, M.; Niculite, C. M.; Gherghiceanu, M.; Lungu, C. P.; Fleaca, C.; Ianchis, R.; Barbut, A.; Grigoriu, C.; Morjan, I. Magnetic iron oxide nanoparticles as drug delivery system in breast cancer Original Research Article Applied Surface Science, Volume 281 ( 2013) 60-65.
- Y. Tai, L. Wang, G. Yan, J.-m. Gao, H. Yu and L. Zhang, Recent research progress on the preparation and application of magnetic nanospheres Polym. Int., 60 ( 2011) 976–994.
- A.-H. Lu, E. L. Salabas and F. Sch¨uth, Angew. Chem., Int. Ed. Magnetic Nanoparticles: Synthesis, Protection, Functionalization, and Application, 46(2007) 1222–1244.
- Rainer Tietze, Stefan Lyer, Stephan Dürr, Tobias Struffert, Tobias Engelhorn, Marc Schwarz, Elisabeth Eckert, Thomas Göen, Serhiy Vasylyev, Wolfgang Peukert, Frank Wiekhorst, Lutz Trahms, Arnd Dörfler, Christoph Alexiou , Efficient drug-delivery using magnetic nanoparticles — biodistribution and therapeutic effects in tumour bearing rabbits Original Research Article Nanomedicine: Nanotechnology, Biology and Medicine, Volume 9, Issue 7,( 2013) 961-971.
- Sophie Laurent, Delphine Forge, Marc Port, Alain Roch, Caroline Robic, Luce Vander Elst, and Robert N. Muller, Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications Chem. Rev. 108( 2008) 2064–2110
- J.L. Arias, V. Gallardo, S.A. Gomez-Lppera, R.C. Plaza, A.V. Delgado, Synthesis and characterization of poly (ethyl-2-cyanoacrylate) nanoparticles with a magnetic core, Journal of Controlled Release 77 (3) (2001) 309–321.
- G. Strom, S.O. Belliot, T. Daemen, D.D. Lasic, Surface modification of nanoparticles to oppose uptake by the molecular phagocyte system, Advanced Drug Delivery Reviews 17 (1) (1995) 31–48.
- K. Morimoto, S. Chono, T. Kosai, T. Seki, Y. Tabata, Design of cationic microspheres based on aminated gelatin for controlled release of peptide and protein drug, Drug Delivery 15 (2) (2008) 113–117.
- Guangshuo Wang, Ying Chang, Ling Wang, Zhiyong Wei, Jianyun Kang, Lin Sang, Xufeng Dong, Guangyi Chen, Hong Wang, Min Qi ,Preparation and characterization of PVPI-coated Fe3O4 nanoparticles as an MRI contrast agent Original Research Article Journal of Magnetism and Magnetic Materials, 340 ( 2013) 57-60.
- Zhongli Lei, Xiaolong Pang, Na Li, Lin Lin, Yanli Li,A novel two-step modifying process for preparation of chitosan-coated Fe3O4/SiO2 microspheres Original Research Article Journal of Materials Processing Technology, Volume 209, Issue 7, 1 ( 2009) 3218-3225
- Jerry S.H. Lee b, Miqin Zhang a,Magnetic nanoparticles in MR imaging and drug delivery Conroy Sun a, Advanced Drug Delivery Reviews 60 (2008) 1252–1265
- B. Feng, R.Y. Hong, L.S.Wang, L. Guo, H.Z. Li ,“Synthesis of Fe3O4/APTES/PEG diacid functionalized magnetic nanoparticles for MR imaging”, Colloids and Surfaces A: Physicochem. Eng. Aspects, 328 (2008) 52–59.
- Michał Moritz, Marek Łaniecki ,SBA-15 mesoporous material modified with APTES as the carrier for2-(3-benzoylphenyl)propionic acid ,Applied Surface Science 258 (2012) 7523– 7529
- S. Asgari, Z. Fakhari, S. BerijaniSynthesis and Characterization of Fe3O4 Magnetic Nanoparticles Coated with Carboxymethyl Chitosan Grafted Sodium Methacrylate , JNS 4 (2014) 55-63
- L. Li , K.Y. Mak , C.W. Leung , K.Y. Chan , W.K. Chan , W. Zhong , P.W.T. Pong, effect of synthesis conditions on the properties of citric-acid coated iron oxide nanoparticles, Microelectronic Engineering 110 (2013) 329–334
- A. Tomitaka, T. Koshi, S. Hatsugai, T. Yamada, Y. Takemura, Journal of Magnetic characterization of surface-coated magnetic nanoparticles for biomedical application , Magnetism and Magnetic Materials 323 (2011) 1398–1403.
- Okassa, L. N.; Marchais, H.; Douziech-Eyrolles, L.; Cohen-Jonathan, S.; Souce, M.; Dubois, P.; Chourpa I, Development and characterization of sub-micron poly(D,L-lactide-co-glycolide) particles loaded with magnetite/maghemite nanoparticles.. Int. J. Pharm. 2005, 302 (1-2), 187.
- D.K. Kim, Y. Zhan, W. Voit, K.V. Rao, M. Muhammed, Synthesis and characterization of surfactant-coated super paramagnetic mono dispersed iron oxide nanoparticles, Journal of Magnetism and Magnetic Materials 225 (2001) 30-36
- M. Yamauraa, R.L. Camiloa, L.C. Sampaiob, M.A. Mac#edoc, M. Nakamurad, H.E. Tomad ,Preparation and characterization of (3-aminopropyl) triethoxysilane-coated magnetite nanoparticles ,Journal of Magnetism and Magnetic Materials 279 (2004) 210–217
- J.M. Rosenholm, M. Lindén, Towards establishing structure-activity relationships for mesoporous silica in drug delivery applications, Journal of Controlled Release 128 (2008) 157–164.
- Bragg W.L. Nature. 1915, 95: 561.
- Y. Jiang, J. Jiang, Q. Gao, M. Ruan, H. Yu, L. Qi, Nanotechnology 19 (2008) 75714.
- Z.M. Rao, T.H. Wu, S.Y. Peng, Acta Phys. Chim. Sin. 11 (1995) 395–399.
- R.D. Waldron, Infrared Spectra of Ferrites ,Phys. Rev. 99 (1955) 1727–1735.
- Z. Xu, Q. Liu, J.A. Finch, Silanation and stability of 3-aminopropyl triethoxy silane on nanosized superparamagnetic particles: I. Direct silanation, Appl. Surf. Sci. 120 (1997) 269–278.
- E. Pretsch, P. Bühlmann, C. Affolter, Structure Determination of Organic Compounds.Tables of Spectral Data, third ed., Springer, Berlin, 2000.

This work is licensed under a Creative Commons Attribution 4.0 International License.