Chemical composition of volatile components, antimicrobial and anticancer activity of n-hexane extract and essential oil from Trachyspermum ammi L. seeds
El-Sayed S. Abdel-Hameed1-3*; Salih A. Bazaid1; Othman Al Zahrani4; Yasser El-Halmouch4,5; Mortada M. El-Sayed3; Eman El-Wakil3
1Natural Products Analysis Laboratory, Faculty of Science, Taif University, Saudi Arabia. 2Chemistry Department, Faculty of Science, Taif University, Saudi Arabia. 3Laboratory of Medicinal Chemistry, Theodor Bilharz Research Institute, Giza, Egypt. 4Biotechnology Department, Faculty of Science, Taif University, Saudi Arabia. 5Botany Department, Faculty of Science, Damanhour University, Damanhour , Egypt.
DOI : http://dx.doi.org/10.13005/ojc/300425
Article Received on :
Article Accepted on :
Article Published : 01 Jan 2015
The aim of this study was to characterize the chemical composition of some volatile components, in vitro antimicrobial and anticancer activity of essential oil and n-hexane extract from Trachyspermum ammi L. (Family Apiaceae). The chemical composition of samples was obtained by GC-MS analysis, the antimicrobial activity was evaluated by disc diffusion method whereas the in vitro anticancer activity was evaluated by sulphorhodamine method. Twenty-three monoterpenoide compounds were identified in the essential oil in which four compounds; γ-terpinene, thymol, P-cymene and β-pinene were the major components of the oil with quantity 266.28, 201.97, 194.91 and 38.49 mg/g oil respectively whereas the other nineteen compounds had quantity < 10 mg/g oil. Twelve monoterpene compounds were identified in the n-hexane extract in which three compounds; thymol, γ-terpinene and P-cymene were the major components of volatile components of the n-hexane extract with quantity 138.85, 56.41 and 32.69 mg/g extract respectively whereas the other nine compounds had quantity < 10 mg/g extract. The essential oil and n-hexane extract exhibited an antimicrobial activity against five microorganisms and an anticancer activity against HepG2. The essential oil showed higher activity than the n-hexane. γ- thymol, terpinene and P-cymene of the two samples play an important role in antimicrobial and anticancer activity. In conclusion, this considered the first report that gave the real quantity of each volatile compound in the essential oil and n-hexane extract of T. ammi. Also, this the first work dealing with the anticancer activity of the two samples in addition to the agreement of antimicrobial activity with previous studies. More safety and toxicological studies will need to be addressed if the essential oil and n-hexane extract of T. ammi are to be used for food preservation or medicinal purposes.
KEYWORDS:Trachyspermum ammi L; essential oil; GC-MS; antimicrobial; HepG2
Download this article as:| Copy the following to cite this article: Abdel-Hameed E. S., Bazaid1 S. A, Zahrani O. A, El-Halmouch Y, El-Sayed3 M. M, El-Wakil E. Chemical composition of volatile components, antimicrobial and anticancer activity of n-hexane extract and essential oil from Trachyspermum ammi L. seeds. Orient J Chem 2014;30(4). |
| Copy the following to cite this URL: Abdel-Hameed E. S., Bazaid1 S. A, Zahrani O. A, El-Halmouch Y, El-Sayed3 M. M, El-Wakil E. Chemical composition of volatile components, antimicrobial and anticancer activity of n-hexane extract and essential oil from Trachyspermum ammi L. seeds. Available from: http://www.orientjchem.org/?p=6610 |
Introduction
The use of natural resources, especially medicinal plants was continued to be excellent sources of phytochemicals for traditional medicines, modern medicines, nutraceuticals, pharmaceutical intermediates, folk medicines, food supplements, and chemical entities for synthetic drugs due to their versatile applications. Natural products from plants continue to be used in pharmaceutical preparations as crude extracts, fractions, or pure compounds. The quest for plants with medicinal properties continues to receive attention as scientists survey plants for a complete range of biological activities, which range from antibiotics to antitumour (1,2).
Essential oils and extracts of aromatic plants have been recognized for many years as a great source of pharmaceutical agents and food additives. They have been employed for a long time in different industries, mainly in perfumes (fragrances and aftershaves), food (as flavourings and preservatives), pharmaceuticals (therapeutic action) and for centuries in traditional medicine. Essential oils are obtained from different plant parts such as flower, buds, seed, leaves and fruits. They are mainly composed of a mixture of volatile low-molecular weight mono- and sesquiterpenes and other isoprenes (3-8).
Trachyspermum ammi L. (Syn. Trachyspermum copticum L.; Family Apiaceae) is an important commercial product for the food and flavouring industry. It is known as a popular aromatic herb and spice that grows in India, Egypt, Persia, Bangladesh, Afghanistan, and Ethiopia. A number of biological actions have been claimed for T. ammi fruits as antispasmodic, stimulant, tonic, digestive, anthelmintic, anticipative, antimicrobial, antiviral, antifilarial, anti-inflammatory, antipyretic, antinociceptive, antifungal, analgesic diuretic and carminative effects, as indicated by various authors and traditional medicine uses. The seeds of this plant contain about 2-4% essential oil that have a biological activity (2, 9-14).
The T. ammi seeds known as Nakhwa or Nankha in Arabic countries and used mainly as a spice and in many folk medicine uses. In Saudi Arabia its traditional name is Nankha that is widely used as a spice component in preparation of Arabic coffee, bread and pastries. Also, mothers used to using its infusion as antispasmodic for either children or adults. Many reports on chemical composition of T. ammi essential oil from different origins revealed that thymol, γ-terpinene p-cymene and carvacrol are the main components (9-11).
To the best of our knowledge, there are no reports on the characterization of volatile components of n-hexane extract of T. ammi seeds. In this study, the authors study the chemical composition of the essential oil and the volatile components of n-hexane extract of T. ammi seeds. Also, the samples were investigated in vitro as anticancer and antimicrobial agents.
Materials and Methods
Chemicals
All solvents, standards and reagents were analytical and HPLC grade from Sigma-Aldrich Chemicals, USA.
Plant material and preparation of essential oil and n-hexane extract
The seeds of plant under investigation; Trachyspermum ammi (Traditionally known as Nankhah) were purchased from the herbal market in Taif governorate, KSA. Voucher specimen (given number TA1) was deposited at the natural products analysis laboratory, faculty of science, Taif University, Taif, KSA. The seeds were ground by electric grinder before the extraction process by n-hexane and hydrodistillation extraction for essential oil.
The essential oil was prepared by hydrodistillation method using Clevenger apparatus. 150 grams of ground seeds were mixed with 1.5 L distilled water in a round flask and connected with the Clevenger apparatus. Using heating mantle at temperature 90 oC, the system was operated for until observing the disappearance of oil collected the vertical receiver-separator column. 2.8 ml (density 0.873 g/mL) clear colorless oil was obtained, dried over sodium sulphate and filtered. The oil was stored at -20 °C in a brown glass vial until investigation.
For the preparation of n-hexane extract, 300 grams of ground seeds were soaked in 1500 ml n-hexane for one week at room temperature with shaking from time to time followed by filtration. The n-hexane was removed under vacuum yielding yellowish green oil. This extraction process was repeated three times. The collected oil (34.5 mL, density 0.919 g/mL) was liquid at room temperature but it was solidified by cooling. The two oils were stored at -20 °C in glass brown vials.
Preparation of standard and sample solutions
Forty nine standard compounds; α-pinene, camphene, benzaldhyde, β-pinene, myrcene, 3-carene, α-terpinene, p-cymene, D-limonene, eucalyptol, γ-terpinene, Sabinene-hydrate, terpineoline, β-Linalool, nonanal, cis-rose oxide, phenyl ethyl alcohol, tras-rose oxide, camphor, menthone, menthol, α-terpineol, verbenone, β-citronellol, cis-citral, carvone, geraniol, trans-citral, citronellyl formate, anethol, thymol, azulene, citronellyl acetate, eugenol, neryl acetate, geranyl acetate, methyl eugenol, β-caryophellene, pentadecane, phenylethyl tigillate, hexadecane, 1-tetradecanol, heptadecane, farnesol, benzyl benzoate, octadecane, nonadecane, 1-eiocosene and heneiocosane were prepared separately by dissolving known weight of each in n-hexane (1 mg/ml) and filtered using membrane disc filter (0.45 µm). For samples, solution (20 mg/ml) of oil was prepared in n-hexane and filtered using membrane disc filter (0.45 µm).
Capillary gas chromatography-mass spectrometry conditions
The GC-MS analysis of the sample and standards were performed using gas chromatograph (GC, Model CP 3800, Varian, California, USA) coupled with a mass spectrometer (MS, Model Saturn 2200, Varian) and auto sampler (Model Combi Pal, Varian) system. The column used for separation was a VF-5 fused silica capillary column (30 m х 0.25 i.d. mm, film thicknesses 0.25 μm, Varian). For MS detector, electron impact (EI) ionization system with ionization energy of 70 eV was used. Trap temperature was set at 170 °C and axial modulation voltage at 4.0 volts. The ions were recorded with mass range 30-450 m/z, solvent delay time 3 min. Helium gas was used as a carrier gas at a constant flow rate of 1 ml/min. Injector and mass transfer line temperature were set at 140 and 280 °C respectively. The optimum conditions for oven temperature were obtained after several trials to get good separated peaks for standards. The program was as follow: 1 min at 50 °C, raised gradually to 120 at 2 °C/min, 120-260 °C at 6 °C/min and held for 1 min, the total run time 60 min. The injection volume of standards and samples was 1 μl with a split ratio 1:20.
Calibration curve and samples analysis
The standards mixture containing 49 compounds was chromatographed using previous analytical conditions. All samples also were analyzed at the same conditions. According to the chromatograms obtaining for samples and review of literatures, another standards mixture containing 15 compounds; α-pinene, camphene, β-pinene, α-phellandrene, α-terpinene, p-cymene, D-limonene, cineol, γ-terpinene, sabinene-hydrate, terpineoline, linalool, α-terpineol, thymol and eugenol was chosen, prepared, and diluted to six different concentrations for establishing calibration curves. A calibration curve of each standard was obtained by plotting the area under peak versus the different concentrations. Chromatograms of the standards and samples were analyzed and processed using Varian MS Workstation software (Service Pack 1, Version 6.5). For samples, known peaks were identified by comparing its retention time (tR) and mass spectrum with standards. Unknown peaks were identified or tentatively identified by matching their mass patterns with Wiley & NIST electronic library and review of literatures.
In vitro microbial sensitivity tests
Essential oil and n-hexane extract were dissolved in a few drops of dimethylsulphoxide (DMSO) and topped up with distilled water to give a stock solution of 100 mg/ml). The stock solution was kept at 4º C. The tested samples were evaluated for their antimicrobial activity against Escherichia coli (ATCC 25922), Pseudomonas aeruginosa ATCC 27853, Enterococcus spp. (ATCC6589), Salmonella enterica, Staphylococcus aureus and Candida albicans. All tested microorganisms were cultured onto a Muller Hinton agar medium which were prepared by adding 38 g of agar powder to one liter of distilled water and the mixture was boiled. The solution was autoclaved at 121 ºC for 20 min and cooled to 50 ºC in a water bath. It was then transferred into sterile Petri dishes.
Disc diffusion method
Bacterial and spore suspension of the tested microorganisms were prepared to a density of 108 cells/ml. The aliquot was spread onto Muller Hinton agar by sterilized cotton swab. Then the plated solid medium was allowed to dry at room temperature (15). Sterile paper discs of 6 mm in diameter were impregnated with 5 μL samples, leaved one hour for dryness and deposited on the agar surface of inoculated plates. Each disc must be pressed down to ensure complete contact with the agar surface. The discs must be distributed no closer than 25 mm from center to center of 150 mm plate. The plates are inverted and placed in an incubator set to 30 °C within 15 minutes after the discs are applied (16). After 48 h the diameters of the zones of complete inhibition are measured, including the diameter of the disc. Discs witted with DMSO was used as negative control.
Anticancer activity
The essential oil and n-hexane extract under study was investigated in vitro towards the liver carcinoma cell line (HepG2) at the National Cancer Institute, Cairo, Egypt, using the method of Skehan et al. (1990) (17). This is a colorimetric assay estimates cell number indirectly by staining total cellular protein with the dye Sulphorhodamine-B (SRB). Different concentrations (0, 12.5, 25, 50 and 100 mg/ml) of oil were added to culture wells. By the end of the experiment, the optical density (O.D.) of each well was measured spectrophotometrically at 564 nm with an ELIZA microplate reader.
The experiment was repeated three times for each cell line. The percentage of cell survival was calculated according to the following equation:
Survival fraction (%) = [O.D. of treated cells/O.D. of control cells] × 100
The IC50 value was calculated from the survival curve of the tumor cell lines by plotting the percent of survival fraction versus different concentrations of oil.
Results & Discussion
Chemical composition of essential oil and n-hexane extract
The GC-MS analysis of the essential oil and n-hexane extract of T. ammi seeds showed the presence only of monoterpenes group and disappearance of sesquiterpenes and aliphatic hydrocarbons (Fig. 1,2 Table 1). Twenty-three monoterpene compounds were identified and quantified in the essential oil. These compounds could be represented by eight subgroups in which the monocyclic monoterpenes, monoterpene phenol, alkylbenzene monoterpene and bicyclic monoterpene subgroups had high quantity; 282.41, 203.74, 194.91 and 52.45 mg/g oil respectively. The other four subgroups; acyclic monoterpene, monocyclic monoterpene alcohol, bicyclic monoterpene alcohol and monocyclic monoterpene acetate showed lower quantity < 10 mg/g oil. Four compounds; γ-terpinene, thymol, P-cymene and β-pinene were the major components of the oil with quantity 266.28, 201.97, 194.91 and 38.49 mg/g oil respectively whereas the other nineteen compounds had quantity < 10 mg/g oil. The presence of the three compounds; γ-terpinene, thymol and P-cymene with high quantity are in good agreement with previous reports on phytochemical investigation of essential oil of this plant (10, 14, 18, 19). In this study, β-pinene had relatively high amount and this was not reported before. In spite of there are some reports concerning the qualitative and quantitative analysis (quantitative analysis based on measuring the area under curve of each compound relative to all compounds areas in the chromatogram) of different compounds of essential oil of this plant, this is the first study which gave the real quantity of each compound based on the calibration curve of the standards.
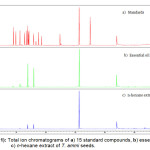 |
Figure1: Total ion chromatograms of a) 15 standard compounds, b) essential oil c) n-hexane extract of T. ammi seeds. |
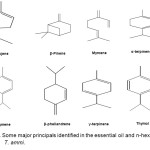 |
Figure2: Some major principals identified in the essential oil and n-hexane extract of T. ammi. Click here to View figure |
Table 1. Qualitative and quantitative chemical composition of essential oil and n-hexane extract of Trachyspermum ammi L. seeds essential oil.
|
No. |
Name |
tR
|
MW[MF] |
mg/g oil |
Class |
|
Essential oil |
|
||||
|
α -thujene1 |
8.73 |
136[C10H16] |
7.32±0.40 |
Bicyclic monoterpene |
|
|
α-pinene2 |
9.07 |
136[C10H16] |
4.61±0.22 |
Bicyclic monoterpene |
|
|
Sabinene1 |
11.00 |
136[C10H16] |
0.98±0.07 |
Bicyclic monoterpene |
|
|
β-pinene2 |
11.25 |
136[C10H16] |
38.49±0.80 |
Bicyclic monoterpene |
|
|
Myrcene1 |
11.89 |
136[C10H16] |
8.67±0.64 |
Acyclic monoterpene |
|
|
α-phellandrene2 |
12.80 |
136[C10H16] |
0.65±0.02 |
Monocyclic monoterpene |
|
|
3-Carene1 |
12.92 |
136[C10H16] |
1.05±0.06 |
Bicyclic monoterpene |
|
|
α-terpinene2 |
13.40 |
136[C10H16] |
7.03±0.28 |
Monocyclic monoterpene |
|
|
p-cymene2 |
13.93 |
134[C10H14] |
194.91±7.74 |
Alkylbenzene monoterpene |
|
|
D-limonene2 |
14.13 |
136[C10H16] |
2.65±0.13 |
Monocyclic monoterpene |
|
|
β -phellandrene1 |
14.21 |
136[C10H16] |
5.22±0.37 |
Monocyclic monoterpene |
|
|
Cineol2 |
14.33 |
154[C10H18O] |
0.39±0.07 |
Bicyclic monoterpene alcohol |
|
|
γ-terpinene2 |
15.96 |
136[C10H16] |
266.28±6.27 |
Monocyclic monoterpene |
|
|
Sabinene hydrate2 |
16.69 |
154[C10H18O] |
0.38±0.03 |
Bicyclic monoterpene |
|
|
Terpineoline2 |
17.55 |
136[C10H16] |
0.58±0.04 |
Monocyclic monoterpene |
|
|
Terpenyl acetate1 |
18.62 |
196 [C12H20O2] |
1.65±0.22 |
Monocyclic monoterpene acetate |
|
|
Trans-2-carene-4-ol1 |
22.68 |
152[C10H16O] |
0.53±0.01 |
Bicyclic monoterpene alcohol |
|
|
Thujol1 |
22.89 |
152[C10H16O] |
0.59±0.09 |
Bicyclic monoterpene alcohol |
|
|
Terpinen4ol1 |
23.77 |
154[C10H18O] |
2.16±0.07 |
Monocyclic monoterpene alcohol |
|
|
α-Terpineol2 |
24.79 |
154[C10H18O] |
1.30±0.10 |
Monocyclic monoterpene alcohol |
|
|
P-thymol1 |
30.71 |
150[C10H14O] |
0.43±0.04 |
Monoterpene phenol |
|
|
Thymol2 |
31.34 |
150[C10H14O] |
201.97±5.55 |
Monoterpene phenol |
|
|
Carvacrol1 |
31.74 |
150[C10H14O] |
1.34±0.16 |
Monoterpene phenol |
|
|
n-hexane extract |
|
||||
|
No. |
Name |
tR
|
MW[MF] |
mg/g extract |
Class |
|
α -thujene1 |
8.74 |
136[C10H16] |
0.75±0.06 |
Bicyclic monoterpene |
|
|
α-pinene2 |
9.08 |
136[C10H16] |
0.43±0.05 |
Bicyclic monoterpene |
|
|
β-pinene2 |
11.26 |
136[C10H16] |
5.65±0.65 |
Bicyclic monoterpene |
|
|
α-phellandrene2 |
11.89 |
136[C10H16] |
1.02±0.11 |
Monocyclic monoterpene |
|
|
p-cymene2 |
13.88 |
134[C10H14] |
32.69±0.60 |
Alkylbenzene monoterpene |
|
|
D-limonene2 |
14.12 |
136[C10H16] |
0.27±0.04 |
Monocyclic monoterpene |
|
|
β -phellandrene1 |
14.22 |
136[C10H16] |
0.68±0.03 |
Monocyclic monoterpene |
|
|
γ-terpinene2 |
15.89 |
136[C10H16] |
56.41±2.25 |
Monocyclic monoterpene |
|
|
Terpenyl acetate1 |
18.66 |
196 [C12H20O2] |
0.62±0.02 |
Monocyclic monoterpene acetate |
|
|
P-thymol1 |
30.71 |
150[C10H14O] |
0.18±0.02 |
Monoterpene phenol |
|
|
Thymol2 |
31.31 |
150[C10H14O] |
138.85±2.68 |
Monoterpene phenol |
|
|
Carvacrol1 |
31.76 |
150[C10H14O] |
0.72±0.13 |
Monoterpene phenol |
|
1: Compounds identified by comparison its mass spectrum with NIST library and review of literatures and quantified from the calibration curve of similar or related standard compound (including molecular weight correction factor).
2: Compounds identified by comparison with standards and quantified from the calibration curve of each standard compound.
The GC-MS analysis of the volatile components of n-hexane extract showed the presence of twelve monoterpene compounds represented by five subgroups; monoterpene phenols, monocyclic monoterpenes, alkylbenzene monoterpene, bicyclic monoterpene and monocyclic monoterpene acetate. Three compounds; thymol, γ-terpinene and P-cymene were the major components of volatile components of the n-hexane extract with quantity 138.85, 56.41 and 32.69 mg/g oil respectively whereas the other nine compounds had quantity < 10 mg/g oil. To the best of our knowledge this study considered the first study concerning the identification of the volatile components of n-hexane extract from T. ammi. The non-volatile components of the n-hexane will be continued to exhaustive investigation by the authors.
Antimicrobial activity
During the past decades and until now there is great interest to use natural anti-microbial essential oils and extracts of various species of medicinal, edible and spice plants as natural agents that considered as non-phytotoxic compounds and potentially effective against microorganisms. Furthermore, with increasing of bacterial resistance to antibiotics, there is a need to investigate more and more essential oils or extracts from plants against a wide range of bacteria, to develop other many natural antimicrobials (20-22).
Antimicrobial activity of the essential oil and n-hexane extract of T. Ammi seeds were varied significantly according to the tested microbes (Fig. 3). The two samples showed antimicrobial activity toward five microbes whereas no activity appeared toward Enterococcus spp. In all responded microbes, the essential oil showed higher activity than the n-hexane extract. The highest action was recorded by essential oil against Salmonella enterica (23 mm) followed by Escherichia coli (22 mm), Candida albicans (21 mm), Staphylococcus aureus (16 mm) and Pseudomonas aeruginosa (7 mm). The n-hexane extract showed activity against the previous five microbes in order; Staphylococcus aureus (10 mm), Escherichia coli (8.7 mm), Salmonella enterica (8 mm) Candida albicans (8.3 mm) and Pseudomonas aeruginosa (6.3 mm). The lethal effect of n-hexane extract on E. coli, S. enterica Candida albicans, P. aeruginosa and C. albicans was varied insignificantly.
The difference in the antimicrobial activities of the two samples is apparently attributable to the differences in its contents of volatile compounds, especially the three major compounds thymol, p-cymene and γ-terpinene. The essential oil had higher quantity of these compounds than the n-hexane extracts. Previous studies have been shown that thymol and its precursors, cymene and terpinene have strong antimicrobial activities (22-24). Some studies have shown that the whole essential oil has a stronger antiseptic activity than the individual major components demonstrating that the minor constituents are also important to the anti-microbial activity and may have a synergistic influence (14, 22, 23, 25, 26).
Anticancer activity
Many reports attributed the anticancer activity of aromatic plants to its content of essential oils as a major phytochemical class (3, 5, 6). The essential oil and n-hexane extract of T. Ammi seeds showed highest anticancer activity toward hepatocellular carcinoma cell line (HepG2) with half-maximal inhibitory concentration (IC50) = 9.57±0.98 and 17.42±1.44 µg/ml respectively (Fig. 4). These values fall within the range of the American National Cancer Institute of (ANCI) criteria which considered the agent is promising when its IC50< 20 µg/ml. The higher activity of essential oil over the n-hexane extract revealed that the anticancer activity is correlated to its high contents of volatile compounds in essential oil. This considered the first report on the anticancer activity of essential oil and n-hexane extract of T. ammi seeds toward HepG2. The anticancer activities of some essential oils were attributed mainly to its main contents of thymol, carvacrol, p-cymene and γ-terpinene as essential oils of Origanum, Thymus and Lippia species (27-32).
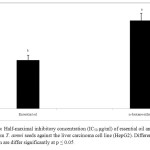 |
Figure3: Half-maximal inhibitory concentration (IC50 μg/ml) of essential oil and n-hexane extract from T. ammi seeds against the liver carcinoma cell line (HepG2). Different letters on the column are differ significantly at p ≤ 0.05. Click here to View figure |
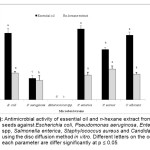 |
Figure4: Antimicrobial activity of essential oil and n-hexane extract from T. ammi seeds against Escherichia coli, Pseudomonas aeruginosa, Enterococcus spp, Salmonella enterica, Staphylococcus aureus and Candida albicans using the disc diffusion method in vitro. Different letters on the column for each parameter are differ significantly at p ≤ 0.05.
|
Conclusion
Essential oils and extracts of aromatic plants have been recognized for many years as a great source of pharmaceutical agents and food additives. The GC-MS analysis of the volatile compounds of essential oil and n-hexane extract of T. ammi seeds showed the presence only of monoterpenes group. Twenty-three monoterpenoide compounds were identified in the essential oil in which four compounds; γ-terpinene, thymol, P-cymene and β-pinene were the major components of the oil with quantity 266.28, 201.97, 194.91 and 38.49 mg/g oil respectively whereas the other nineteen compounds had quantity < 10 mg/g oil. Twelve monoterpene compounds were identified in the n-hexane extract in which three compounds; thymol, γ-terpinene and P-cymene were the major components of volatile components of the n-hexane extract with quantity 138.85, 56.41 and 32.69 mg/g extract respectively whereas the other nine compounds had quantity < 10 mg/g extract. This is the first study which gave the real quantity of each compounds based on the calibration curve of the standards. Antimicrobial activity of the essential oil and n-hexane extract of T. ammi seeds were varied significantly according to the tested microbes. The two samples showed antimicrobial activity against five microbes; Salmonella enterica, Staphylococcus aureus, Pseudomonas aeruginosa, Staphylococcus aureus and Escherichia coli, while no activity observed against Enterococcus spp. The essential oil and n-hexane extract of T. ammi seeds showed high anticancer activity against HepG2 with IC50 = 9.57±0.98 and 17.42±1.44 µg/ml respectively. To the best of our knowledge, this study considered the first report on the anticancer activity of the T. ammi. The higher antimicrobial and anticancer activity of essential oil than the n-hexane extract could be correlated with the high content of thymol, p-cymene and γ-terpinene in the essential oil. The results of this study suggest that the essential oil and n-hexane extract of T. ammi could be used as an alternative to synthetic bactericides for using in the food industry and medicinal purposes after more safety and toxicological studies.
Acknowledgment
The author is very grateful to Taif University, Kingdom of Saudi Arabia for supporting this work.
Competing interests
The Author has declared that no competing interests exist.
References
- E.S. Abdel-Hameed, S.A. Bazaid, and M.Salman, Characterization of the phytochemical constituents of Taif rose and its antioxidant and anticancer activities. BioMed Res. Inter., Article ID 345465, 13 pages. http://dx.doi.org/ 10.1155/ 2013/345465, 2013.
- R. Khana, M. Adila, M. Danishuddina, P. K. Vermab and A.U. Khana, In vitro and in vivo inhibition of Streptococcus mutans biofilm by Trachyspermum ammi seeds: An approach of alternative medicine. Phytomedicine, 2012, 19, 747-755.
- F. Bakkali, S. Averbeck, D. Averbeck, and M. Idaomar, Biological effects of essential oils: a review. Food Chem. Toxicol., 2008, 46, 446-475.
- B. Adorjan and G. Buchbauer, Biological properties of essential oils: an updated review. Flavour Fragr. J., 2010, 25, 407-426.
- H.C. Baser, and G. Buchbauer, Handbook of essential oils science, technology, and applications. CRC Press Taylor & Francis Group, New York, United States of America 2010.
- P. Bhardwaj, U. Alok and A. Khanna, In vitro cytotoxicity of essential oils. Inter. J. Res. Pharm. Chem., 2013, 3, 675-681.
- A.E. Hamdy, H. E. Ahmed and S.Takayuki, Bioactivity of essential oils and their volatile aroma components: Review. J. Ess. Oil Res., 2012, 24(2), 203–212.
- B. Andrade, L. Barbosa, I. Probst and A. Júnior, Antimicrobial activity of essential oils. J. Ess. Oil Res., 2014, 26(1), 34-40.
- S. Morshed, M. K. Alam, A. Begum, S.K. Shahriar, N. Sharmin and S. Akther: Physicochemical Properties and Chemical Constituents of Oil from Joan Seed (Trachyspermum ammi L.). J. Environ. Sci. Nat. Res., 2012, 5(2), 15- 21.
- M. Mahboubi and N. Kazempour: Chemical composition and antimicrobial activity of Satureja hortensis and Trachyspermum copticum essential oil. Iran. J. Micribiol., 2011, 3(4), 194-200.
- I. Rasooli, M. H. Fakoor, D. Yadegarinia, L. Gachkar, A. Allameh and M. B. Rezaei: Antimycotoxigenic characteristics of Rosmarinus officinalis and Trachyspermum copticum L. essential oils. Inter. J. Food Microbiol., 2008, 122, 135-139.
- R. Velazhahan, S. Vijayanandraj, A.P. Vijayasamundeeswari, R. Samiyappan, T. Iwamoto, B. Friebe and S. Muthukrishnan, Detoxification of aflatoxins by seed extracts of the medicinal plant, Trachyspermum ammi (L.) Sprague ex Turrill- Structural analysis and biological toxicity of degradation product of aflatoxin G1. Food Control 2010, 21, 719–725.
- T. Kaur, R. Bijarnia, S. Singla and C. Tandon, In vivo efficacy of Trachyspermum ammi anticalcifying protein in urolithiatic rat model. J. Ethnopharmacol., 126, 459-462 (2009).
- M. Moazeni, M. J. Saharkhiz and A. A. Hosseini, In vitro lethal effect of ajowan (Trachyspermum ammi L.) essential oil on hydatid cyst protoscoleces. Vet. Parasitol., 2012, 187, 203-208.
- V. Lopez, A.K. Jager, S. Akerreta, R.Y. Cavero and M.I. Calvo, Pharmacological properties of Anagallis arvensis L. (“scarlet pimpernel”) and Anagallis foemina Mill. (“blue pimpernel”) traditionally used as wound healing remedies in Navarra (Spain). J. Ethnopharmacol., 2011, 134, 1014-1017.
- D. Kalemba and A. Kunicka, Antibacterial and antifungal properties of essential oils, Curr Med Chem, 2003, 10, 813-829.
- P. Skehan, R. Storeng, D. Scudiero, A. Monks, I. McMahon and D. Vistica, New colorimetric cytotoxicity assay for anticancer drug screening. J. Nat. Cancer Inst., 1990, 82, 1107-1112.
- M. H. Boskabady, S. Alitaneh and A. Alavinezhad, Carum copticum L.: A herbal medicine with various pharmacological effects. BioMed Res. Inter., Article ID 569087, 11 pages (2014).
- J. Mimija and J. E. Thoppil, Essential oil composition of Trachyspermum ammi (L) Sprague from South India. Ind. J. Pharm. Sci., 2002, 64, 250-251.
- G. J. Nychas, C. C. Tassou and P. Skandamis, Antimicrobials from herbs and spices. In S.m. Roller (Ed.), Natural antimicrobials for the minimal processing of foods (pp. 176-200). New York: Woodhead Publishers/CRC Press 2003.
- S. Paul, R.C. Dubey, D.K. Maheswari and S.C. Kang, Trachyspermum ammi (L.) fruit essential oil influencing on membrane permeability and surface characteristics in inhibiting food-borne pathogens. Food Control 2011, 22, 725-731.
- S. Burt, Essential oils: their antibacterial properties and potential applications in foods: a review. Int. J. Food Microbiol., 2004, 94, 223–253.
- G.R. Goudarzi, M.J. Saharkhiz, M. Sattari and K. Zomorodian, Antibacterial activity and chemical composition of ajowan (Carum copticum benth. & hook) essential oil. J. Agric. Sci. Technol. 2011, 13, 203–208.
- D. Trombetta, F. Castelli, M.G. Sarpietro, V. Venuti, M. Cristani, C. Daniele, A. Saija, G. Mazzanti and G. Bisignano, Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother., 2005, 49, 2474–2478.
- A. Mourey and N. Canillac, Anti-Listeria monocytogenes activity of essential oils components of conifers. Food Control 2002, 13, 289–292.
- O.R. Karami, M. Khodaverdi and A.F. Ali, Antibacterial effect of effective compounds of Satureja hortensis and Thymus vulgaris essential oils against Erwinia amylovora. J. Agric. Sci. Technol. 2010, 12, 35–45.
- A. I. Hussain, Characterization and biological activities of essential oils of some species of Lamiaceae (PH.D. Thesis) Department of Chemistry and Biochemistry, Faculty of Sciences, University of Agriculture, Faisalabad, Pakistan 2009.
- S. Sertel, T. Eichhorn, P. K. Plinkert and T. Efferth, Cytotoxicity of Thymus vulgaris Essential Oil Towards Human Oral Cavity Squamous Cell Carcinoma. Anticancer Res. 2011, 31, 81-88.
- R.P. Ferraz, D.S. Bomfim, N.C. Carvalho, M.B. Soares, T.B. Silva, W.J. Machado, A.P. Prata, E.V. Costa, V.R. Moraes, P.C. Nogueira and D.P. Bezerra, Cytotoxic effect of leaf essential oil of Lippia gracilis Schauer (Verbenaceae). Phytomedicine, 2013, 20, 615-621.
- M. Nikolic, J. Glamoclija, I.C. Ferreira, R.C. Calhelha, A. Fernandes, T. Markovic, D. Markovic, A. Giwelie and M. Sokovic, Chemical composition, antimicrobial, antioxidant and antitumor activity of Thymus serpyllum L., Thymus algeriensis Boiss. and Reut and Thymus vulgaris L. essential oils. Ind. Crop. Prod., 2014, 52, 183-190.
- A. Jaafari, H. Mouse, E.M. Rakib, L. Mobarek, M. Tilaoui, C. Benbakhta, A. Boulli, A. Abbad and A. Zyad, Chemical composition and antitumor activity of different wild varieties of Moroccan thyme. Rev. Bras. Farmacogn., 2007, 17, 477-491.
- J. Al-Kalaldeh, R. Abu-Dahab and F. Afifi, Volatile oil composition and antiproliferative activity of Laurus nobilis, Origanum syriacum, Origanum vulgare, and Salvia triloba against human breast adenocarcinoma cells. Nutr. Res., 2010, 30, 271-278.

This work is licensed under a Creative Commons Attribution 4.0 International License.









