A convenient route for synthesis and antimicrobial evaluation of bis (diimino benzothiazolo pyrimido pyrimidines)
Balasaheb D. Kalyankar, Prashant N. Ubale and Sambhaji P. Vartale*
PG Resarch Center, Department of Chemistry, Yeshwant Mahavidyalaya Nanded-431602 (MS) India
DOI : http://dx.doi.org/10.13005/ojc/300448
Article Received on :
Article Accepted on :
Article Published : 14 Nov 2014
Guanidine hydrochloride (1) on treatment with bis(methylthio)methylene malonitrile (2) in N,N-dimethyl formamide (DMF) and catalytic amount of anhydrous potassium carbonate gives diimino pyrimido pyrimidine (3). The later were further reacted with various substituted 2-amino benzothiazoles (4) to gives bis (diimino benzothiazolo pyrimido pyrimidines) (5a-g). All these synthesized compounds were screened for their antimicrobial activity.
KEYWORDS:Bis(methylthio)methylene malononitrile; Guanidine hydrochloride; N; N-dimethyl formamide (DMF) and Potassium carbonate
Download this article as:| Copy the following to cite this article: Kalyankar B. D, Ubale P. N, Vartale S. P. A convenient route for synthesis and antimicrobial evaluation of bis (diimino benzothiazolo pyrimido pyrimidines)”. Orient J Chem 2014;30(4). |
| Copy the following to cite this URL: Kalyankar B. D, Ubale P. N, Vartale S. P. A convenient route for synthesis and antimicrobial evaluation of bis (diimino benzothiazolo pyrimido pyrimidines)”. Orient J Chem 2014;30(4). Available from: http://www.orientjchem.org/?p=5330 |
INTRODUCTION
Pyrimido pyrimidine and its derivatives are interesting class of nitrogen containing heterocyclic compounds which are widely found in bio-organic and medicinal chemistry with application in drug discovery. Pyrimido [4,5-d] pyrimidines, pyrido [2,3-d] pyrimidines are important class of annulated uracils which have biological importance1 because of their connection with purine pyrimidine system2 . Some of them exhibit significant biological and pharmacological activities such as antifolate activity3, antibacterial activity4-7, tyrosine kinase activity8. Numerous reports have appeared in the literature for preparation of fused heterocycles. Derivatives of pyrimido pyrimidine are useful as bronchodilators9, vasodilators10, antiallergic11 and hypersensitive agent12, more over they display potent inhibitory properties regarding tyrosine kinase domain of epidermal growth factor receptor13. Various researcher reported pyrimido pyrimidine explore the antitumor14, antiviral15, antioxidant16, antifungal17 and hepatoprotective activities18.
A survey of literature reveals that few references are available on the synthesis and biological activity of heterocycles containing a benzothiazole fused with pyrimidine ring19-22. Patrick Jimonet and his research group23 reported the synthesis and pharmacological activity of 3-substituted -2-imino benzothiazolines. These compounds were found to be three times more potent than 6-(trifluoromethoxy)-2-benzothiazolamine (Riluzole), a blocker of excitatory amino acid mediated neurotransmission. These observations stimulated us to considerable interest for the synthesis of new compounds in which the imino pyrimidine ring is fused through a nitrogen atom with another biological active nucleus such as benzothiazole. Recently our research group reported synthesis of pyrimido thiazine and its derivatives24-26.
The present review is devoted exclusively to the synthesis of diimino pyrimido pyrimidine and its reaction with 2-Amino benzothiazoles. In present manuscript we emphasize the synthetic routes to heterocyclic compounds via diimino pyrimido pyrimidine with evaluation of their anti microbial activity.
MATERIAL AND METHOD
All the chemicals used in the present study are of analytical grade purchased from Himedia chemical co. All reactions were monitored by thin layer chromatography, were carried out on 0.2 mm silica gel-C plates, the spots were examined under UV light chamber. Melting points of synthesized compounds were determined by Electrothermal IA 9000 SERIES digital melting point apparatus and were uncorrected. Solvents were purified according to standard procedure. Infrared spectra were recorded in Nujol or as potassium bromide pallets on infrared spectrophotometer, nuclear magnetic resonance spectra were obtained on Brukner advance spectrophotometer 400 MHz, Mass spectra were recorded on FT-VC-7070 H Mass spectrometer using the EI technique at 70 eV. All the reactions were carried out under ambient atmosphere. Elemental analysis was performed on a Heraeus CHN-O rapid analyser.
GENERAL PROCEDURE
2,6-dihydro-2,6-diimino-4,8-bis(methylthio)-1H-pyrimido[1,2-a]pyrimidine-3,7-dicarbonitrile (3)
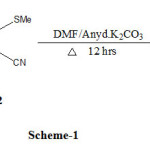
A mixture of guanidine hydrochloride (1) (0.01 mol) and bis(methylthio)methylene malononitrile (2) (0.02 mol) in 20 ml of N,N-dimethyl formamide (DMF) and anhydrous potassium carbonate (10 mg) was refluxed for 12 hours. After completion of reaction, the reaction mixture was cooled to room temperature and poured in to ice cold water. The seperated solid product was filtered , washed with water and recrystalized from N,N-dimethyl formamide-ethanol mixture to give pure (3).
Yellow powder, Yield 62%, M.P. 190-192 °C. IR (KBr/cm-1) 3416 cm-1 (=NH) , 3380 cm-1 (-NH), 2205 cm-1 (CN), 1680 cm-1 (C=N-): 1HNMR (400 MHz,DMSO-d6): δ = 2.1 (s, 1H, -NH), 2.7 ( s, 6H, SCH3), 8.1 (s, 2H,=NH): EI-MS(m/z: RA% ): 303 (M+). Anal. Calcd for C11H9N7S2:C:43.56, H:2.97, N:32.34, found: C:43.31, H:2.84, N:32.21.
Diimino bis benzothiazolo pyrimido pyrimidines (5a-g)
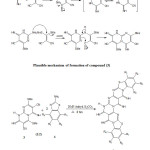
A mixture of (3) (0.001 mol) and independently varios substituted 2-amino benzothiazoles (4) like 2-amino benzothiazole, 2-amino-6-methyl benzothiazole, 2-amino-6-methoxy benzothiazole, 2-amino-6-bromo benzothiazole, 2-amino-4,6-dimethyl benzothiazole, 2-amino-5,6-dichloro benzothiazole, 2-amino-5-nitro benzothiazole (0.002 mol) in 10 ml of N,N-dimethyl formamide (DMF) and anhydrous potassium carbonate(6 mg) was refluxed for 8 hours. The reaction mixture was cooled to room temperature and poured in to ice cold water. The separated solid product was filtered , washed with water and recrystalized from N,N-dimethyl formamide-ethanol mixture to give pure (5a-g).
5a
Brown powder, Yield 68%, M.P. 215°C. IR (KBr/cm-1) 3450 cm-1 (=NH), 3365 cm-1 (NH), 1690 cm-1 (C=N). 1HNMR (400 MHz,DMSO-d6): 6.2-6.7 (m, 8H, Ar-H), 8.5 (s, 4H, =NH): EI-MS (m/z: RA% ): 507 (M+). Anal. Calcd for C23H13N11S2 C: 54.43, H: 2.56, N: 30.37, found; C: 54.27, H: 2.51, N: 30.15.
5b
Brown powder, Yield 71%, M.P. 205°C. IR (KBr/cm-1) 3432 cm-1 (=NH), 3372 cm-1 (NH), 1660 cm-1 (-C=N-). 1HNMR (400 MHz,DMSO-d6): δ = 2.2 ( s, 6H, Ar-CH3), 6.1-6.8 (m, 6H, Ar-H), 9.1 (s, 2H, =NH): EI-MS (m/z: RA% ): 535 (M+). Anal. Calcd for C25H17N11S2 C:56.07, H:3.17, N:28.73; found: C:56.01, H:3.08, N:28.45.
5c
Brown powder, Yield 66%, M.P. 224°C. IR (KBr/cm-1) 3440 cm-1 (=NH), 3342 cm-1 (NH), 1667cm-1 (-C=NH-): 1HNMR (400 MHz,DMSO-d6): δ = 3.6-3.8 ( s, 6H, -OCH3), 6.8-7.5 (m, 6H, Ar-H), 8.8 (s, 2H, =NH): EI-MS (m/z: RA% ): 567 (M+). Anal. Calcd for C25H17N11O2S2 C:52.91, H:2.99, N:27.16; found; C:52.77; H:2.82; N:27.03.
5d:
Yellow powder, Yield 62%, M.P. 219°C. IR (KBr/cm-1) 3426 cm-1 (=NH), 3354 cm-1 (NH), 1678 cm-1 (-C=N-): 1HNMR (400 MHz,DMSO-d6): δ = 7.0-7.7 (m, 6H, Ar-H), 9.2 (s, 4H, =NH): EI-MS (m/z: RA% ): 665 (M+). Anal. Calcd for C23H11N11S2Br2 C:41.50; H:1.65; N:23.15; found; C:.41.34; H:1.51; N:23.07.
5e
Yellow powder, Yield 72%, M.P. 230°C. IR (KBr/cm-1) 3442 cm-1 (=NH), 3377 cm-1 (NH), 1673 cm-1 (-C=N-): 1HNMR (400 MHz,DMSO-d6): δ = 2.3 ( s, 12H, -CH3), 6.2-6.6 (m, 4H, Ar-H), 8.8 (s, 4H, =NH): EI-MS (m/z: RA% ): 563 (M+). Anal. Calcd for C27H21N11S2 C:57.54, H:3.73, N:27.35; found; C:57.36, H:3.67, N:27.25.
5f
Brown powder, Yield 58%, M.P. 236°C. IR (KBr/cm-1) 3446 cm-1 (=NH), 3350 cm-1 (NH), 1680 cm-1 (-C=N-): 1HNMR (400 MHz,DMSO-d6): δ = 6.5-7.8 (m, 4H, Ar-H), 9.5 (s, 4H, =NH): EI-MS (m/z: RA% ): 645 (M+). Anal. Calcd for C23H9N11S2Cl4 C:42.79, H:1.39, N:23.87; found: C:42.72, H:1.24, N:23.62.
5g
Brown powder, Yield 65%, M.P. 210°C. IR (KBr/cm-1) 3420 cm-1 (=NH), 3340 cm-1 (NH), 1675 cm-1 (-C=N-): 1HNMR (400 MHz,DMSO-d6): δ = 6.8-7.8 (m, 6H, Ar-H), 9.6 (s, 4H, =NH): EI-MS (m/z: RA% ): 597 (M+). Anal. Calcd for C23H11N13O4S2 C:46.23, H:1.84, N:30.48; found; C:46.08, H:1.59, N:30.32.
 |
Scheme 1 Click here to View scheme |
 |
Scheme 2 Click here to View scheme |
|
Comp. No. |
R1 |
R2 |
R3 |
R4 |
|
5a |
-H |
-H |
-H |
-H |
|
5b |
-H |
-CH3 |
-H |
-H |
|
5c |
-H |
-OCH3 |
-H |
-H |
|
5d |
-H |
-Br |
-H |
-H |
|
5e |
-H |
-CH3 |
-H |
-CH3 |
|
5f |
-H |
-Cl |
-Cl |
-H |
|
5g |
-H |
-H |
-NO2 |
-H |
RESULT AND DISCUSSION
Our approach to synthesize bis (diimino benzothiazolo pyrimido pyrimidines), we have performed efficient reaction to connect guanidine hydrochloride (1) and bis (methylthio) methylene malononitrile (2) were condensed in N,N dimethyl formamide and catalytic amount of anhydrous potassium carbonate to afford (3) Scheme-1
The compound possesses replacable active methyl thio group which is activated by nitrogen atom, electron withdrawing cyano group. When compound (3) (1mole) was condensed independently with 2-amino benzothiazole, 2-amino-6-methyl benzothiazole, 2-amino-6-methoxy benzothiazole, 2-amino-6-bromo benzothiazole, 2-amino-4,6-dimethyl benzothiazole, 2-amino-5,6-dichloro benzothiazole, 2-amino-5-nitro benzothiazole (4) in N,N dimethyl formamide (DMF) and catalytic amount of anhydrous potassium carbonate to obtain diimino bis benzothiazolo pyrimido pyrimidines (5a-g)
The identy of the product was determined by elemental analysis, IR,1HNMR, MASS spectral studies. IR spectram of compound shows absorption band in the region 2250-2180 cm-1for (CN). 1HNMR spectra of compound exhibit singlet peak in the region of 8.0-9.5 ppm due to (=NH) proton. The MASS spectra shows molecular ion peak which is corresponds to the molecular weight of respective compounds. Spectral studies of all compounds shows that compounds were stable and do not exhibit any tautomerism. The elemental analysis values are in good agreement with theoretical data. All the compounds were screened for their antibacterial and antifungal activities.
ANTIMICROBIAL ACTIVITY
The synthesized compounds were evaluated for their antifungal and antibacterial activities against species Candida albicus, E.coli, S.typhy, S.aureus, B.subtilis by paper disk diffusion method. These compounds were dissolved in dimethyl sulphoxide. Incubation period for fungi was 4 days and for bacteria was 24 hours. The newly synthesized compounds shows zone of inhibition 5-18 mm in diameter where as standard streptomycin exhibit zone of inhibition 18-22 mm in diameter against E.coli, S.typhi, S.aureus and B.subtilis. Standard amphotericin B shows zone of inhibition 18 mm in diameter against Candida albicus. Among all the newly synthesized compounds, 5f (16 11 10 13 16 mm) and 5g (12 13 11 14 12 mm) showed higher zone of inhibition against E.coli, S.typhi, S.aureus, B.subtilis and Candida albicus due to presence of di- Cl and NO2 groups.
Table 1. Antimicrobial activity of compound (5a-g)
|
|
Zone of inhibition in mm |
||||
|
Compounds
|
Bacteria |
fungi |
|||
|
E.Coli |
S.typhi |
S.aureus |
B.subtilis |
Candida Albicus |
|
|
5a |
– |
08 |
05 |
07 |
09 |
|
5b |
05 |
07 |
09 |
06 |
13 |
|
5c |
– |
– |
11 |
12 |
12 |
|
5d |
10 |
06 |
15 |
08 |
06 |
|
5e |
08 |
05 |
09 |
– |
12 |
|
5f |
16 |
11 |
10 |
13 |
16 |
|
5g |
12 |
13 |
11 |
14 |
12 |
|
Positive control |
18 | 18 | 22 | 20 | 18 |
|
Streptomycin |
Amphoterecin B |
||||
CONCLUSION
In conclusion, we have synthesized diimino pyrimido pyrimidine (3), bis (diimino benzothiazolo pyrimido pyrimidines) (5a-g) have been reported first time and obtained by simple route using mild condition with good yield. These newly synthesized compounds were screened for their antimicrobial activities, compound 5d and 5f posseses very good antibacterial and antifungal activity. Hence the importance of such work lies in the possibility that the new compounds might be more effictive drug against bacteria and fungi, which would be helpful in designing more potent antibacterial and antifungal agent for therapeutic use.
ACKNOWLEDGEMENT
The authors are grateful to principal, Yeshwant Mahavidyalaya, Nanded, for providing laboratory facilities, and the Director, Indian Institute of Chemical Technology, Hyderabad, for providing spectra.
REFERENCES
- Srivastava S. K., Haq W., Chauhan P. M. S., Bioorg. Med. Chem. Lett, 1999, 9, 965.
- (a) Lunt E, In Barton, D. Ollis, W. Eds , Comprehensive organic chemistry, pergamon press, Oxford, 1974, vol 4, p .493 (b) Brown J D, In Katrizky, A R Rees, C W Eds, Comprehensive Heterocyclic Chemistry, Pergamon Press, Oxferd, 1984, vol 3, p (c) Clercq E D, Braaerts R J, Biol. Chem, 1987, 262, 14905.
- Grivski E M, Lee S, Sigel C W, Duch D S and Nichol C A, J. Med. Chem., 1980, 23, 327.
- Matsumoto J and Minami S, J. Med. Chem.,1975, 18, 74.
- Mont N, Teixido J, Borrell J I and Oliver Kappe C, Tetrahedron Lett., 2003, 44, 5385.
- Oakes V and Rydon H N, J.Chem. Soc., 1956, 10, 4433.
- DeGraw J I, Kisliuk R L, Gaumont Y and Baugh C M, J. Med. Chem., 1974, 17, 470.
- Thompson A M, Rewcastle G W, Bridges A J, Fry D W, Kraker A J, Denny W A and McMichael A, J. Med. Chem., 1995, 38, 3780.
- Coates W J, European patent 351058, Chem. Abstr., 1990, 113, 40711, Ramsey A A. U. S. Patent 3830812 FMC Corp., Chem. Abstr., 1974, 81, 134174.
- Taylor E C, Knopf R J, Meyer R F, Holmes A, Hoefle M L, J. Am. Chem. Soc.,1960, 82, 5711. Figueroa Villar J D, Carneiro C L, Cruz E R, Heterocycles, 1992, 34, 891.
- Kitamaru N, Onishi A, European patent 163599, Chem. Abstr., 1984, 104, 186439.
- Raddatz P, Bergmann R, German patent 360731, Chem. Abstr., 1988, 109, 54786.
- Rewcastle G W, Bridge A J, Fry D W, Rubin J R, Denny W A, J. Med. Chem., 1997, 40 1820.
- Sanghvi Y S, Larson S B, Matsumoto S S, Nord L D, Smee D F, Willis R C, Avery T H, Robins R K, Revankar G R, J.Med. Chem., 1989, 32, 629-637.
- Tenser R B, Gaydos A K, Hay A, Antimicrob Agents Chemother, 2001, 45, 3657-3659.
- De la Cruz J P, Carrasco T, Ortega G, Sanchez De la Cuesta F, Lipids, 1992, 27, 192- 194.
- Sharma P, Rane N, Gurram V K, Bioorg. Med. Chem. Lett., 2004, 14, 4185-4190.
- Ram V J, Goal A, Sarkhel S, Maulik P R, Bioorg. Med. Chem. Lett.,2002,10, 1275-1280.
- Singh A and Bhal A, Ind. J. Chem., 1969, 7, 302.
- Reimlinger H, Peiren M A, Merenyi R, Chem. Ber., 1975, 108, 3894.
- Gurinder J S D, Dorcas I O, Scheinmann F, Bates P A, Hursthouse M B, J. Chem. Soc. Perkin Trans I, 1988, 2993.
- Hataba A A, Fikry R M and Moustafa H Y, J. Ind. Chem. Soc., 1997, 74, 818.
- Jimonet P, Audiau F, Barreau M, Blanchard J C, Boireau A, Bour Y, Coleno M A, Doble A, Doerflinger G, Huu C D, Donat M H, Duchesne J M, Ganil P, Gueremy C, Honore C, Just B, Kerphirique R, Gontier S, Hubert P, Laduron P M, Le Blevec J, Meunier M, Miquet J M, Nemecek C, Pasquet M, Piot O, Pratt J, Ratund J, Reibaud M, Stutzmann J M and Mignani S, J. Med. Chem. 1999, 42, 2828.
- Sirsat Shivraj B, Vartale Sambhaji P, Organic chem. Curr res, 2012, 1, 5.
- Sirsat S B, Halikar N K, Kalyankar M B and Vartale S P, journal of pharm. Res, 2012, 5, 2700.
- Sambhaji P Vartale, Shivraj B Sirsat and Nilesh K Halikar, Heterocycl. Commun. 2013, 19 (3), 215-218.

This work is licensed under a Creative Commons Attribution 4.0 International License.









