Synthesis and in vitro biodegradation of poly(ethylene adipate-co-D,L-lactic acid) copolymers (PLEA)
Bakouri Hichem, Guemra Kaddour* and Boukhouya Imene
Laboratory of Macromolecular Physical Organic Chemistry Djillali Liabes University, BP89 City El Arbi Ben Mhidi Sidi Bel Abbes, (Algeria).
DOI : http://dx.doi.org/10.13005/ojc/300318
Article Received on :
Article Accepted on :
Article Published : 17 Sep 2014
The present research mainly focused on the synthesis and study of bio degradation of poly (ethylene adipate-co-D,L-lactic acid). PLEA were prepared via ring opening polymerization from D,L-lactide and poly(ethylene adipate). PLEA was characterized by 1H NMR spectra and DSC; results showed that those properties showed high dependence on its composition.In vitro degradation behaviors of PLEA have been systematically investigated up to 12 weeks in phosphate buffer saline solution at 37 °C. The weight and polymer molecular weight were measured as a function of degradation time. Bacterial degradation was completed by investigating the physico-chemical properties using spectroscopy FTIR and DSC.
KEYWORDS:Ethylene adipate; D; L-Lactide; Bio degradation; Bacteria; physico-chemical properties
Download this article as:| Copy the following to cite this article: Hichem B, Kaddour G, Imene B. Synthesis and in vitro biodegradation of poly(ethylene adipate-co-D,L-lactic acid) copolymers (PLEA). Orient J Chem 2014;30(3). |
| Copy the following to cite this URL: Hichem B, Kaddour G, Imene B. Synthesis and in vitro biodegradation of poly(ethylene adipate-co-D,L-lactic acid) copolymers (PLEA). Orient J Chem 2014;30(3). Available from: http://www.orientjchem.org/?p=4699 |
INTRODUCTION
The synthesis routines of poly(lactic acid) (PLA) could be directly divided into polycondensation reaction from lactic acid and ring opening polymerization (ROP) from lactides, a coordination-insertion mechanism has been proposed and widely accepted [1.2].
Microorganisms degrading PLA identified in literature are limited to Actinomycetes Amy-colatopsis type, and a bacterium, Bacillus Brevis. However, despite of the growing interests for its biomedical applications, many properties of PLA products still fall short of those required for some potential applications, due to its lackage of toughness and impact resistance [3.4.5].
The poly(ethylene adipate) (PEA), exhibits excellent flexibility, whereas its mechanical strength is relatively poor and cannot meet various requirements due to its relatively low melting temperature (60 °C) [6.7].
Furthermore, as one of the most common aliphatic polyesters prepared from diacids and diols, PEA is also expected to be an economically competitive biodegradable polymer for its potent to be degraded and assimilated completely by microorganisms, as well as its low cost [8.9].
Structural changes or composition modifications have been regarded as effective approaches to improve the properties of the polymers and promote its application to a much broader range [4.10.11].
Taking into consideration the difference in chain flexibility and physical properties between PLA and PEA, it seems of interest to prepare PLEA copolymer with hard PLA and soft PEA segments sharing the merits of PLA such as biocompatibility and biodegradability and PEA such as flexibility and thermal properties. Up to now, few related works have been reported on such a copolymer.
Moreover, taking into the consideration of excellent biodegradable performance of PLA and PEA, the PLEA copolymers are expected to be good alternatives for the non-degradable polymers in various applications.
In this article, we describe the synthesis of PLEA copolymers with various compositions, but the aim of this work was mainly dedicated to bacterial degradation of PLEA at 37 °C. Moreover, in vitro degradation behaviors of the sample in phosphate buffer saline solution (PBS) solution at 37 °C were investigated in detail, with emphasis on change of the molecular weight, physico-chemical properties, weight and morphologies of sample during degradation.
EXPERIMENTAL
Materials
Adipic acid (AA) (p.a. Fluka), ethylene glycol (EG) (p.a. Fluka), D,L-lactide and stannous octoate were acquired from Aldrich. We investigated four different polymer formulations: PEA, PLEA75/25, PLEA55/45 and PDLLA. PLEA with lactic/ethylene adipate (LA/EA) molar ratio of 75/25 (PLEA75/25), 55/45 (PLEA55/45) and Poly(D,L-lactide) (PDLLA) were synthesized via ring opening polymerization. Average molecular weights, melting and glass transition temperatures of these polyesters are shown in Table 1.
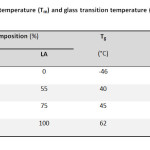 |
Table1: Molecular weight, melting temperature (Tm) and glass transition temperature (Tg) of the polyesters Click here to View table |
Synthesis of hydroxyl terminated PEA
PEA prepolymer was synthesized by a two-stage preparation procedure, including esterification and polycondensation, AAand EG with a feeding molar ratio 1/2 were added into the reactor, with 0.1 wt% stannous chlorides as catalyst. The reactor was evacuated and then filled with nitrogen for several times, in order to remove all of the oxygen. Then, the mixture was heated to 200 °C under stirring of 60 rpm with reaction pressure of 0.35 MPa for 4 h to eliminate the water generated.
The PEA prepolymer was removed, cooled, and milled. The PEA prepolymer was dissolved in chloroform and precipitated with methanol, then filtered and dried under vacuum at 40 °C to a constant weight for use.
Synthesis of PLEA copolymers via ROP
Different molar ratio of hydroxyl terminated PEA prepolymer and D,L-lactide mixed with stannous octoate (1wt%) were added into a oneneck flask; after degassing, the flask was sealed under vacuum and the polymerization was proceeded at 120 °C for 24 h. The copolymers were dissolved in chloroform initially and then precipitated by adding excessive amount of cold methanol under stirring. Finally, the precipitants were filtered and washed by methanol, then dried under vacuum at 40 °C up to constant weight. The synthesis routine was shown in Figure 1.
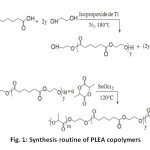 |
Fig1: Synthesis routine of PLEA copolymers Click here to View Figure |
Microorganisms and Inoculums Preparation
Bacteria
The following bacterial strains cited in the ISO 846 standard [12] were used in the bio-degradation tests: Bacillus subtilis and Pseudomonas aeruginosa. These bacterial strains were supplied by the German DSMZ (Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH) in lyophilized form and distributed separately in sterile glass tanks.
In oculums Preparation
At first, each bacterial strain was rehydrated separately. In a second step, a specific amount of hydrated fungus was inoculated into a solution composed of 250 ml of basal liquid medium for bacteria which was incubated at 37 °C during 72 h.
The composition of the basal liquid medium for bacteria is: 1.0 g of NH4NO3; 0.7 of KH2PO4; 0.3 g of K2HPO4; 0.5 g of (MgSO4, 7 H2O); 0.5 g of NaCl; 0.01 g of (FeSO4, 7 H2O); and 1L of distilled water. The pH was adjusted to 6–6.5 by NaOH solution at 0.01 mol/L.
Incomplete agar medium for the growth test
Add to mineral salts solution, enough agars to obtain a concentration of 20 g/L. The mixture is boiled with stirring.
Full agar medium for the bacterio static effect
Add to incomplete agar medium, enough glucose to obtain a concentration of 30 g/L.
Methods
Biodegradation kineticsofPLEA
All the experiment vessels and mineral media were sterilized by autoclaving at 121 °C at a pressure of 1 bar for 20 min or by decontamination using a 0.5% sodium hypochlorite solution.
Testing growth
Samples polymers are see ded with a bacterial strain(Pseudomonasaeruginosa)in the presence of in complete nutrient medium(free from carbon source). Bacterial growth can then be established at the expense of the polymer,in fact,if the polymeris not a nutrient so bacteria can not grow [12].
Bacterio static effects
The sample is subjected tothis time bacterial strain in the presence of a complete nutrient medium(with a carbon source). Evenif the plastic contains nonutrients,bacteria can grow and meta bolites can attackthe material. The reaction medium is identical with the previous by the presence of glucose(at 30 g/L)as the carbon source.
Hydrolytic Degradation Test
Hydrolytic degradation experiments of copolyesters were carried out in a phosphate buffer solution (PBS, pH=7.4) at 37 °C. The samples were stored in a thermostatical water bath for up to 12 weeks. At predetermined degradation time intervals, the samples were isolated from the medium, washed with purified water and then dried under vacuum at room temperature for 48 h before analysis.
REULTS AND DISCUSSION
Chemical structure and compositions
Figure 2a illustrates the 1H NMR spectra of hydroxyl terminated PEA prepolymer synthesized via polycondensation reaction. The proton resonance signals arise at =1.64 (H2), 2.34 (H1), 3.72 (H6), 3.82 (H4), 4.21 (H5), and 4.27 ppm (H3), and the assignment of the signals is shown in accordance with the digital numbers marked in the structural formula of the PEA. Among the proton signals, =3.72 (H6), 3.82 (H4), 4.21 ppm (H5) were reasonably attributed to those protons in the end groups.
The relative integrity value of the methylene proton signals could be used to calculate the degree of polymerization (Dp) and number-average molecular weight (Mn, NMR) of PEA prepolymer by using [4]
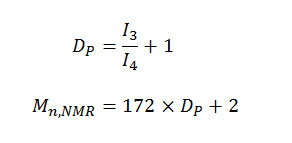
where I3 and I4 represent the intensity of methylene peak of EG located in the internal and end of PEA prepolymer’s molecular chain, 172 and 2 the masses of repeating unit of EA segments and hydrogen atoms at the end of PEA prepolymer’s molecular chain, respectively. The value of Mn, NMR was 1278 g/mol, corresponding closely to Mn, viscosimetry 1319 g/mol.
Typical 1H NMR spectrum of PLEA copolymers was illustrated in Figure 2b. It was found that all the intrinsic proton resonance signals for PEA and PLA homo polymers were all detected at chemical shifts of =2.37 (H1), 1.68 (H2), 4.28 ppm (H3) for PEA, as well as 5.10 (H4) and 1.52 ppm (H5) for PLA, respectively. Moreover, other new signals were also found at chemical shifts at =4.38 (H6), the assignment of which was marked in the sequence structure of PLEA copolymer in.
 |
Fig2: 1H NMR spectra of PEA prepolymer (a), and PLEA copolymers (b) Click here to View Figure |
Thermal properties of PLEA copolymers
As known to us, since the copolymerization reaction alters the composition as well as the regularity of the polymer chains, the thermal behaviors are thus changed. The content of the segments is always considered to be a key ingredient, which decides the properties of the copolymers. Therefore, it is of importance to investigate the dependence of thermal properties on the content of the segments [2.13]. DSC analysis of the PLEA copolymers with different compositions was showed in Table 1.
It is obvious that Tm and Tg of PLLA homopolymer are much higher than that of PEA homopolymer, while Tm and Tg of PLEA copolymers are all intermediate between the two. It suggests that the glass transition and melting behavior of PLEA copolymers were dominated by LA segments, while EA segments remained amorphous in the PLEA copolymers.
As shown it Table 1, it could be found that Tg and Tm observed gradually increases with more LA segments incorporated into the polymer chains, from PLEA-1 to PLEA-2. This tendency is attributed to the inferior flexibility of LA segments compared with EA segments, which could be drawn by the evidence that Tg of PDLLA homo polymer (62 °C) is much higher than that of PEA homo polymer (-46 °C). The increase in Tm and Tg of the PLEA copolymers with higher LA content (or lower EA content) could attribute to the two possible functions of EA segments. The first one is that EA segments serve as soft blocks to increase the chain flexibility of PLEA copolymers, and the other one is acting as heterogeneous blocks to decrease the chain regularity of PLEA copolymers. That is why the decrease in EA content results in increasing crystallization ability of the LA segments, which further contributes to the increase of Tm.
Testing growth
In poor medium (without glucose), visual examination after a month on the polymer samples exposed to a suspension of bacteria (Figure 3) shows a low bacterial growth visible tothe naked eye. The material does not contain nutrients. The masses of the copolymer before and after the invasion of microbial tests are virtually equal; bacterial degradation under these conditions does not exist.
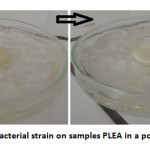 |
Fig3: Growth of a bacterial strain on samples PLEA in a poor medium at 37 °C Click here to View Figure |
The bacteri ostatic effect
The visual examination of the samples of polymers exposed to a bacterial suspension in complete medium (Figure 4) demonstrates a strong visible to the naked eye bacterial growth. So there total lack of bacteri ostatic effect for PLEA, it does not inhibit the growth of bacteria metabolizing glucose. The copolymer apparently is not causing toxicity towards bacteria.
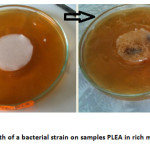 |
Fig4: Growth of a bacterial strain on samples PLEA in rich media at 37 °C Click here to View Figure |
Evolution of physico-chemical properties
Two types of mixtures were prepared for this test to distinguish the purely bacterial degradation and chemical hydrolysis. The biodegradation tests were performed in a biotic medium (polymer inoculated by bacteria), and also in an abiotic medium (not inoculated by bacteria) to investigate the effect of the hydrolysis degradation. In a biotic medium, a process of bio degradation can occur; in an abiotic one, only chemical hydrolysis can happen.
At predetermined time intervals of degradation residues visible samples, are isolated periodically clamp undergoes various washings with water, dried in a desiccators at room temperature for 48 h and then weighed to determine the weight loss and the reduced viscosity of the sample, characterization by DSC and FTIR spectroscopy were determined at the same time intervals.
Test hydrolytic degradation «abiotic medium»
The degradation characteristics of the copolymers (PLEA-1, PLEA-2) were evaluated by measuring the weight loss and decrease in reduced viscosity. For this purpose, the copolymer is pre-weighed and then placed in 25 ml of PBS pH 7.4 at 37 °C.Phosphate Buffer Solution (PBS) was prepared by dissolving the mixture of KH2PO4 (4.710 g) and Na2HPO4 (19,778 g) in 1 L of sterile distilled water.
Evolution of the residual mass
Changes in the molecular weight and the mass of the samples were monitored throughout the process of hydrolytic degradation. The effects of hydrolytic degradation are measured and expressed as a variation of the residual mass as a function of time in Figure 5.
Moreover, the results also indicated that the degradation rate of the copolymer increases with increasing PEA content. This phenomenon has been attributed to the morphology of the copolymers. The copolymer with PLA low (PLEA-1) was amorphous and water easily penetrated the mass of the copolymer, so that the rate of degradation of the samples was fast, but in the PLA alone, the water was difficult to into the polymer as it is semi-crystalline and the rate of degradation of the samples was low.
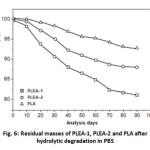 |
Fig. 6: Residual masses of PLEA-1, PLEA-2 and PLA after hydrolytic degradation in PBS Click here to View Figure |
Evolution of the reduced viscosity of PLEA
Figure 6 shows a drastic decrease of the reduced viscosity in abiotic medium during the first 17 days.
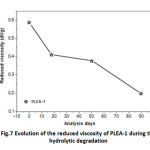 |
Fig7: Evolution of the reduced viscosity of PLEA-1 during the hydrolytic degradation Click here to View table |
Infrared Analyses
Figure 7 shows the FTIR spectra in the attenuated total reflection mode of PLEA at its initial state (t=0) and after 90 days of degradation in abiotic media. Peak positions and assignments for PLEA initial are: ν (–CH–) stretch at 2,997 and 2,985 cm-1; carbonyl ester (C=O) at 1,759 cm-1, δ (–CH–) deformation at 1,383 and 1,365 cm-1, ν (–C–O–) stretch at 1,186, 1,134, 1,091 and stretch ν (–C–C–) at 870 cm-1.
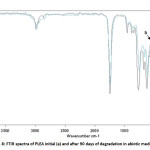 |
Fig8: FTIR spectra of PLEA initial (a) and after 90 days of degradation in abiotic media (b) Click here to View Figure |
Spectra comparison before and after degradation showed a decrease in the band at 1,186 cm-1 that corresponds to ν (–C–O–) stretch. The decrease in the band at 1,186 cm-1 is due to a cleavage in polymer chains by chemical hydrolysis of ester bonds [14.15]. This step of non-random degradation that occurs in the amorphous region of polymer (PEA) easily reached induces a decrease in the molecular weight.
Test degradation by bacteria «biotic medium»
Thermal behavior of PLEA during the biodegradation
Figures 8 show the evolution of Tg and Tm of PLEA given at three-time analysis 0, 50 and 90 days.
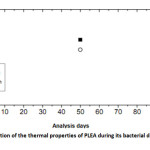 |
Fig9: Evolution of the thermal properties of PLEA during its bacterial degradation Click here to View Figure |
Several changes are observed in the thermal properties of PLEA during the bio degradation process: decreases of Tg from 44.9 to 39.2 °C and that of Tm from 190.2 to 163.7 °C between the beginning of the test and the day 90. The fact that Tg decreased indicates that the PLEA undergone a physical degradation throughout the experiment conducted. The occurred damage in the amorphous phase and the formation of low molecular weight fragments, which are easily crystallized, lead to an increase of the degree of crystallinity in the earliest days of the bio degradation. Then, this percentage crystallinity starts later to decrease which means that the crystalline regions start to be damaged because of the bio assimilation of the small fragments by the microorganisms. Gattin and al. [16] had investigated on the bio degradation of a polylactic acid-based material by microorganisms in a liquid medium. They found the Tg values decreased strongly throughout the experiment of bio degradation and with increasing time, the extent of the degradation was characterized by simultaneous decreases of Tg and percentage crystallinity.
Infrared Analyses
PLEA fragments, recovered after 50 and 90 days in biotic mediums were analyzed by FTIR (Figure 9).Spectra comparison before and after degradation showed the formation of a new band at 1,600 cm-1 that corresponds to carboxylate ions in the spectrum of the biotic media. The appearance of carboxylate ions at the 1,600 cm-1 position is due to microorganisms which consume lactic acid and its oligomers on the surface and leave carboxylate ions at the chain end [17].
The first step in the bio degradation is a chemical hydrolysis in the middle of the polymer chain with the oligomer in the migration medium; the second stage corresponds to a bacterial mineralization at the ends of strings. The PLEA therefore undergoes a profound change in its chemical structure activity precedes significant mineralization (chemical hydrolysis and bio assimilation are not simultaneous).
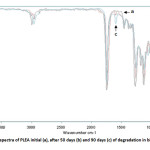 |
Fig10: FTIR spectra of PLEA initial (a), after 50 days (b) and 90 days (c) of degradation in biotic media Click here to View Figure |
A series of PLEA copolymers with various compositions was synthesized from hydroxyl terminated PEA prepolymer and D,L-lactide with stannous octoate (SnOct2) as catalyst via ROP.
DSC analysis demonstrated that the crystal structures in PLEA copolymers were dominated by LA segments, while EA segments remained amorphous due to the existence of ester exchange reaction during the polymerization process. Moreover, Tm and Tg increased with less EA segments incorporated into the PLEA.
The evaluation of the ability of bacteria to use a polymer as a carbon source has been demonstrate by testing growth on solid media, performed at 37°C, show that the conformations adopted by the polymer seem more accessible to enzymes microorganisms.
After defining the bacteria degrading copolymer, monitoring changes in the physico-chemical properties of the polymer during the degradation and the time was made.
In the case of PLEA, at the beginning the monomers and monoesters (formed by hydrolysis) are bio assimilated by microorganisms then a long hydrolysis of ester bands occurs due to the temperature and the humidity, then the oligomer fragments formed by extended hydrolysis are bio assimilated by microorganisms. During these two steps, hydrolysis and bio assimilation, both molecular weights and glass transition decrease (degradation happens at the level of ester bonds and at chain-ends).
REFERENCES
- Garlotta, D. A.,literature review of poly (lactic acid).J. Polym. Environ.,9(2):63–84 (2001)
- Li, B.; Chen, S.C.; Qiu, Z.C.;et al.,Synthesis of poly(lactic acid-b-p-dioxanone) block copolymers from ring opening polymerization of p-dioxanone by poly(L-lactic acid) macroinitiators. Polym. Bull.,61(2):139–146 (2008)
- Mehta, R.; Kumar, V.; Bhunia, H.; Upadhyay, S.N.,Synthesis of poly (lactic acid): a review. J. Macromol. Sci. Polym. Rev., 45(4):325–349 (2005)
- Zeng, J.B.; Li, Y.D.; Zhu, Q.Y.;et al.,A novel bio- degradable multiblock poly (ester urethane) containing poly (L-lactic acid) and poly (butylene succinate) blocks. Polymer.,50(5):1178–1186 (2009)
- Ba, C.Y.; Yang, J.; Hao, Q.H.; et al.,Syntheses and physical characterization of new aliphatic triblock poly (L-lactide-b-butylene succinate-b-L-lactide)s bearing soft and hard biodegradable building blocks. Biomacromolecules.,4(6):1827–1834 (2003)
- Kim, S.W.; Lim, J.C.; Kim, D.J.; Seo, K.H.,Synthesis and characteristics of biodegradable copolyesters from the transesterification of poly (butylene adipate-co-succinate) and poly (ethylene terephthalate). J. Appl. Polym. Sci.,92(5):3266–3274 (2004)
- Monvisade, P.; Loungvanidprapa, P.,Synthesis of poly (ethylene adipate) and poly (ethylene adipate-co-terephthalate) via ring-opening polymerization. Eur. Polym. J.,43:3408–3414 (2007)
- Atkins, T.W.,Biodegradation of poly (ethylene adipate) microcapsules in physiological media. Biomaterials.,19(1–3):61–67 (1998)
- Kim, M.N.; Kim, K.H.; Jin, H.J.;et al., Biodegradability of ethyl and n-octyl branched poly (ethylene adipate) and poly (butylene succinate). Eur. Polym. J.,37(9):1843–1847 (2001)
- Kramer, J.W.; Treitler, D.S.; Dunn, E.W.; et al.,Polymerization of enantiopure monomers using syndiospecific catalysts: a new approach to sequence control in polymer synthesis. J. Am. Chem. Soc.,131(44):16042–16044 (2009)
- Pfeifer, S.; Lutz, J.F.,A facile procedure for controlling monomer sequence distribution in radical chain polymerizations. J. Am. Chem. Soc.,129(31):9542–9543 (2007)
- ISO 846, Plastics—evaluation of the action of microorganisms. International Organization for Standardization, Geneva (1997)
- Deng, L.M.; Wang, Y.Z.; Yang, K.K.; et al., A new biodegradable copolyester poly (butylene succinate-co-ethylene succinate-co-ethylene terephthalate). Acta. Mater., 52(20):5871–5878 (2004)
- Hakkarainen, M.; Karlsson, S.; Albertsson, A.C.,Polymer.,41:2331 (2000)
- Khabbaz, F.; Karlsson, S.; Albertsson, A.C.,J. Appl. Polym. Sci.,78:2369 (2000)
- Gattin, R.; and al., Comparative biodegradation study of starch- and polylactic acid-based materials. Journal of Polymers and the Environment.,2001. 9(1): p. 11-17
- Khabbaz, F.; Karlsson, S and Albertsson, A.C., Py-GC/MS an effective technique to characterizing of degradation mechanism of poly (L-lactide) in the different environment. Journal of Applied Polymer Science.,2000. 78(13): p. 2369-2378

This work is licensed under a Creative Commons Attribution 4.0 International License.









