Synthesis and Characterization of Cobalt(II), Nickel(II) and Copper(II) Complexes with Nitrogen-Oxygen Donor Ligand
B. K. Rai1, Vineeta Singh2, Puja Sinha3, S. N. Vidyarthi4, Shashi Bhushan Sahi5, Ashok Pandey4 and Amit4
1Department of Chemistry, L. N. T. College, B. R. A. Bihar University, Muzaffarpur, India.
2B.N.R. +2 Govt. School, Guljarbag, Patna, India
3FNS Academy, +2 Govt. School, Guljarbag, Patna, India
4University Department of Chemistry, J. P. University, Chapra, India
5M. H. Degree College, Tarwara, Siwan, India
DOI : http://dx.doi.org/10.13005/ojc/300363
Article Received on :
Article Accepted on :
Article Published : 14 Jul 2014
The complexes of ML2 were synthesized where M= Co(II), Ni(II) and Cu(II) and L= 2-Butyl thioquinazoline 4 (3H)- semicarbazone (BTQS). The ligand have been synthesized by condensation of thioquinazoline-4(3H)-one with semicarbazide hydrochlande characterized by molar mass, elemental analysis, Infrared spectra, electronic spectra, magnetic susceptibility and molar conductance measurements .The ligand BTQS acts as neutral bidentate chelating agent and coordinated to metal ions through azomethine nitrogen and carbonyl oxygen of semicarbazone moiety. The remaining Coordination centres are satisfied by anions such as Cl-, Br-, I- and NO3-.
KEYWORDS:Schiff base / BTQS / Co(II); Ni(II) and Cu(II) / Complexes
Download this article as:| Copy the following to cite this article: Rai B. K, Singh V, Sinha P, Vidyarthi S. N, Sahi S. B, Pandey A, Amit. Synthesis and Characterization of Cobalt(II), Nickel(II) and Copper(II) Complexes with Nitrogen-Oxygen Donor Ligand. Orient J Chem 2014;30(3). |
| Copy the following to cite this URL: Rai B. K, Singh V, Sinha P, Vidyarthi S. N, Sahi S. B, Pandey A, Amit. Synthesis and Characterization of Cobalt(II), Nickel(II) and Copper(II) Complexes with Nitrogen-Oxygen Donor Ligand. Orient J Chem 2014;30(3). Available from: http://www.orientjchem.org/?p=4194 |
INTRODUCTION
Transition metal complexes of Schiff base have been intensely investigated by Coordination chemists due to their interesting structural properties and their wide ranging applications1-5, The coordination ability of Schiff bases with transition element, particularly with first row, afford the study of the metals in such system. Keeping the above facts in mind and in continuation of our recent work6-21 we now, present the results of our investigations on the synthesis, spectral and the theoretical studies of Co(II), Ni(II) and Cu(II) complexes with ligand, 2-butyl thioquinazoline 4(3H) semicarbazone (BTQS).
EXPERIMENTAL
All the reagents herein used were of analytical grade. The metal contents of the complexes were estimated using standard procedures22. The IR spectra (Table-2) were recorded on Perkin-Elmer-577 spectrophoto-meter using KBr disc. Magnetic susceptibility were measured on Gouy balance using Hg[CO(NCS)4] as a calibrant . Electronic spectra were recorded on Systronics conductivitymeter model 303 using DMF as a solvent.
Preparation of the Ligand
Ethanolic solution of 2-butyl thioquinazoline 4(3H) one (0.01m) was treated with semicarbazide hydrochloride (0.01 m) dissolved in minimum amount of sodium acetate in ethanol. The resulting mixture was heated for 2 and half hour with occassional stirring. The product crystallized from ethanol and dried. m.p. 263±1oC. Yield 65%.
Preparation of the complexes
The complexes of Cu(II), Co(II) and Ni(II) have been prepared by reacting solution of metal halides with solutions of the ligand BTQS in molar ratio 1:2. The solid coloured complexes which was separated on cooling were filtered, washed with ethanol, dried in oven. Yield in all cases 60-65%.
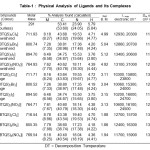 |
Table1: Physical Analysis of Ligands and Its Complexes
|
RESULTS AND DISCUSSION
I.R. Spectra
IR spectra of the ligand and complexes were recorded on Perkin Elmer Spectrophotometer Model 577 using KBr disc.
The IR spectra of the ligand exhibit strong and broad band at 1560 cm-1 assignable23-25 to nC=N. The band is shifted to lower wave number after complex formation, proposes involvement of azomethine N in the bonding with metal ions. The appearance of a band in far IR region at 425-395 cm-1. In the complexes assignable24-26 to nM-N. The IR spectra of the ligand exhibits strong and broad band at 1760 cm‑1 assignable25-28 to nC=O. This band undergoes to shift after complex formation, proposes coordination of metal ion through carbonyl oxygen. It is further supported by the appearance of a new far IR band at 525-505 cm-1 in the complexes which is assignable24-26 to nM-O. The linkage with halogen is indicated by the appearance of another band in the far IR region 320-280 cm‑1 assigned24-26 to nM-X (X= Cl–, Br– or I–). The coordination with halogen is supported by the low molar conductivity of the complexes in the range 11.2-13.7 ohm-1 cm2 mol-1.
Nitrate complexes show characteristic medium intensity bands at 1360 and 1180 cm-1 with a separation 140 cm-1 due to monodentate linkage of nitrate groups. Combination band at 1660 cm-1 and 1640 cm-1 with a separation of 20cm-1 conforming the monodentate behavior of the nitrate29 group.
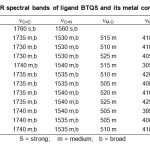 |
Table2: IR spectral bands of ligand BTQS and its metal complexes. Click here to View table |
Electronic Spectra and Magnetic Susceptibility of the Complexes
The Co(II) complexes exhibit two spectral bands in the region at 13400-12860 cm-1 and 2100-20300 cm-1 assigned to the transitions, 4T1g(F) ® 4A2g(F) and 4T1g(F) ® 4T1g(P) proposing octahedral30,31 geometry. The proposed geometry of Co(II) complexes are further supported by the high magnetic susceptibility value in the range 4.92-5.12 B.M. The Ni(II) complexes display three spectral bands in the regions, 10600-10000 cm-1 16100-15300 cm-1 and 24700-23600cm-1 assigned to the transitions, 3A2g(F) ® 3T2g(F), 3A2g(F) ® 3T1g(F) and 3A2g(F) ® 3T1g(P) respectively, suggested octahedral geometry30,34 for Ni(II) complexes. The propsed geometry of Ni(II) complexes is further supported by the magnetic susceptibility value in the range 3.04-3.15 B.M. The Cu(II) complexes exhibit two spectral bands in the regions, 11700-12400 cm-1 and 16300-15700cm-1 assigned to the transitions, 2Eg ® 2T2g and charge transfer band suggested octahedral30,35 geometry for the Cu(II) complexes. The magnetic susceptibility value of Co(II) complexes lies in the range 1.86-1.94 B.M.
Molar Conductance Measurement
Molar conductance value of the complexes of Co(II), Ni(II) and Cu(II) were found to be in the range 11.2-13.7 ohm-1 cm2mol-1 in the DMF which proposes their non-electrolytic nature. The molar conductance values also supported the structure assigned on the basis of physico-chemical and spectroscopic measurements.
![Fig.1 [M(BTQS)2 ] M = Co(II) and Ni(II); X = Cl-, Br-, I- or NO3-. M = Cu(II); X = Cl-, Br- or NO3-.](http://www.orientjchem.org/wp-content/uploads/2014/07/Vol30_No3_Synt_Rai_fig1-150x150.jpg) |
Fig1: [M(BTQS)2 ]M = Co(II) and Ni(II); X = Cl–, Br–, I– or NO3–. M = Cu(II); X = Cl–, Br– or NO3–. |
CONCLUSIONS
Thus on the basis of above mentioned studies, it is proposed that the ligand BTQS acts in a neutral bidentate manner and coordinates is proposed through azomethine nitrogen and carbonyl oxygen of semicarbozone moiety which is also supported by the literature. The remaining positions of metal ions are satisfied by negative ions such as Cl–, Br–, I– and NO3–. The geometry of Co(II), Ni(II) or Cu(II) complexes are proposed to be octahedral on nature as given in structure-I.
Acknowlwdgement
The author is thankful to the University Grant Commission (U.G.C.) New Delhi for financial support for this work.
REFERENCES
- L. David, M. Rasu, O. Cozar, D. Rusu, M. Todica and C. Balam, J. Mol. Struct., 482-483, 149(1999).
- W. Plass, A. Pohlmann and Y. Hakan-Peter, J. Inorg. Biochol; 80, 181(2001).
- N. Galic, B. Peric, B. Kojic-Prodic and Z. Cimerman; J. Mol. Struct;; 559, 187(2001).
- Vivekanand and N.S. Bhandari, Asian J. Chem; 19, 4225(2007).
- J. Singh and P. Singh, Bioinorg Chem; Appl; Article ID, 104549(2012).
- Rai B.K., Vidyarthi S.N., Sinha Puja, singh Kalyan Chandra, Shahi Shashi Bhushan and Ojha Jayvir Sharan, OrientJ. Chem. 28, 1365(2012).
- Rai B.K., Vidyarthi S.N., Amit, Singh Rabindra, Bhardwaj Nitish and Ojha Avinash, Orient J. Chem; 28, 1403 (2012).
- Rai B.K. Sinha Puja, Vidyarthi S.N. and Singh Vineeta, Asian J. Chem., 23, 4629 (2011).
- Rai B. K., Singh Vineeta, Vidyarthi S. N. and Sinha Puja, Asian J. Chem., 23, 4638 (2011).
- Rai B.K., Vidhyarthi S.N., Kumari Punam, Kumari Suman, Kumari Lakshmi and Rajkishore Singh, Asian J. Chem, 25, 941(2013).
- Rai B.K., Anand Rahul, Asian J. Chem., 25, 480(2013).
- Rai B.K., Thakur Amrita and Divya, Asian J. Chem., 25, 583 (2013).
- Rai B.K., Kumar Arun, Asian J. Chem., 25, 1187 (2013).
- Rai B.K. and J. Indian Chem., soc; 90, 105 (2013).
- Rai B.K., Vidyarthi S.N., Sinha Puja, Singh Vineeta and Kumar Sanjiv, Orient J. Chem., 29, 271 (2013).
- Rai B.K., Kumar Sanjay, Anand Rahul and Pandey Ashok, Orient J. Chem., 29, 655 (2013).
- Rai B.K, Singh Rabindra, Anand Puja, Singh Sunil Kumar and Amit, Orient J. Chem., 29, 753 (2013).
- Rai B.K., Vidyarthi S.N., Prakash Om and Baluni, Akhilesh Orient J. Chem; 29, 801 (2013).
- Choudhry Chitranjan Prasad, Sharma S.P., Rai C.L. and Rai B.K., Orient J. Chem., 29, 963 (2013).
- Rai B.K., Kumari Rachna, Orient J. Chem., 29, 1163 (2013).
- Rai B.K. and Kumar Arun, Orient J, Chem, 29, 1187 (2013).
- Vogel, Textbook of Quantitative Chemical Analysis, Revised by Mendham J., Denny R.C., Barenes J.P. and Thomas M., Pearson Education (2008).
- Mishra A.P., Purwar H. and Jain R.K., Biointerface Res. Appl. Chem, 2, 291 (2012).
- Silverstein Rober and Webster Francis, “Spectrometric Identification of Organic Compounds”, 6th Ed. John-Wiley and Sons, (2008).
- Kemp William, Organic Spectroscopology, 3rd Edn., Polgrove, New York, (2008).
- Ferraro J.R., ‘Low Frequency Vibration of Inorganic and Coordination Compound”, Plenum Press, New York.
- Reddy K. Laxman and Upendra S., Indian J. Chem., 39 A, 1202 (2000).
- Rao C.N., Chemical application of IR Spectroscopy, Academic Press, New York, 206(1963).
- Addition C. C. Logan N. Wallwork S. C. and Barmer D. C., Quart. Rev., (1971).
- Lever, A.B.P. Inorganic Electronic spectroscopy, Elsevier, Amsterdam, 395(1968).
- Mane P.S. Shirodhar S.G., Arbad B.R. and Chandekar T.K., Indian J. Chjem., 40A, 648(2000).
- Figgis B.N., Introductio to Ligand Field, Wiley Eastern Ltd., New Delhi, 279(1976).
- Carlin R.L. and Van Dryneveldt a.J. Magnetic Properties of Transition metal compounds, springer verlag, NewYork(1997).
- Tahir A.K., Shivanjal, Nafees J. and Shoukat K., Indian J. Chem., 39A, 450(2000).
- Mishra A.P., Khare M and Gauta, S.K., Synth. React. Inorg. Met. Org. Chem. 32, 1485(2002).

This work is licensed under a Creative Commons Attribution 4.0 International License.









