Structural and Spectroscopic Aspects of Schiff Base Metal Complexes of Cobalt(II), Nickel(II) and Copper(II)
B.K. Rai1*, Puja Sinha2, Vineeta Singh3, S.N. Vidhyarthi4, Amit4, Ashok Pandey5, and Shashi Bhusan shahi4.
1Department of Chemistry, L. N. T. College, B. R. A. Bihar University, Muzaffarpur,
2 F N S Academy +2 Govt. School, Guljarbagh, Patna
3 B N R +2 Govt. School, Guljarbagh, Patna
4 University Department of Chemistry, J. P. University, Chapra
5M. H. Degree College, Tarwara, Siwan
DOI : http://dx.doi.org/10.13005/ojc/300366
Article Received on : April 15, 2014
Article Accepted on : May 24, 2014
The complexes of Co(II), Ni(II) and Cu(II) with Schiff base 2-butyl thioquinazoline 4(3H) thiosemicarbazone were synthesized. The general formulae of the complexes are of the type {M(L)2X2], L=2 – butyl thioquinazoline 4(3H) thiosemicarbazone; x = Cl-, Br-, I- and NO3-. Elemental analyses and spectral (IR, electronic) studies of the synthesized complexes suggest the presence of octahedral, environment around the central metal ion. These complexes were also subjected to study their antimicrobial screening against, Gram positive bacteria Candida albicans and gram negative bacteria Escherichia coli by disc diffusion technique.
KEYWORDS:Schiff bases;BTQT ; Complex / Co(II); Ni(II) and Cu(II); antimicrobial screening
Download this article as:| Copy the following to cite this article: Rai B. K, Sinha P, Singh V, Vidhyarthi S. N, Amit, Pandey A, Shahi S. B. Synthesis and Characterization of Cobalt(II), Nickel(II) and Copper(II) Complexes with Nitrogen-Oxygen Donor Ligand. Orient J Chem 2014;30(3). |
| Copy the following to cite this URL: Rai B. K, Sinha P, Singh V, Vidhyarthi S. N, Amit, Pandey A, Shahi S. B. Synthesis and Characterization of Cobalt(II), Nickel(II) and Copper(II) Complexes with Nitrogen-Oxygen Donor Ligand. Orient J Chem 2014;30(3). Orient J Chem 2014;30(3). Available from: http://www.orientjchem.org/?p=4207 |
INTRODUCTION
Thiosemicarbazone used as suitable ligand due to variable bonding abilities and several biocidal importance such as inhibitory action antiviral, antibacterial and anti-fungal activity1-9. Based on the above biocidal importance of thiosemicarbazone and in continuation of our earlier recent work10-23 on the synthesis and characterization of Schiff base metal complexes with nitrogen and sulphur containing Schiff base, we report here the synthesis and antimicrobial evaluation of complexes of Co(II), Ni(II) and Cu(II)with the ligand, 2 butyl-thioquinazoline 4(3H) thiosemicarbozone.
EXPERIMENTAL
The chemical and reagent were used as obtained from commercial supplies without further purification. The analysis of carbon, hydrogen and nitrogen as well as metal ions were performed using standard procedure24. IR spectra were recorded in KBr pellets in 4000-200 cm-1 region on Perkin Elmer-577 spectrophotometer. Electronic spectra were recorded on Cary-2390 spectrophotometer in the 10000-25000 cm-1. Magnetic susceptibility of the complexes were done on a Gouy balance using Hg[Co(NCS)4] as a calibrant. Molar conductivity of the Complexes were done at room temperature on Systronics conductivity meter model 303 using DMF as a solvent. Antimicrobial activity of the ligand and the complexes was carried out against E. coli and S. aureus by disc diffusion method.
Preparation of the ligand.
A solution of thiosemicarbazide (0.01 mol) in 25 ml in ethanol was added to 2-butyl thioquinazoline 4(3H) one (0.01 m) dissolved in ethanol (25 ml) with occasional stirring. The reaction mixture was heated under reflux on a water bath for 4 h. After reducing the solvent volume to Ca . 30 ml and cooling to room temperature, the colourless solid obtained was filtered, washed with ethanol and dried in a oven. Yield-70% m.p.- 3160C ± 10C
Synthesis of complexes
The complexes were prepared by reating appropriate metal halide/nitrate with the ethanolic solution of the ligand in molar ratio 1:2. The resulting mixture was refluxed for 3-4 h. On cooling to room temperature the coloured complexes were precipitated out. It was filtered, washed with hot water, ethanol and finally dried in oven yield 60-65%.
RESULTS AND DISCUSSION
In order to give conclusive evidence about the structure of the metal complexes, the main IR bands of the metal complexes, the main IR bands were compared with those of the free ligands. The IR spectra of the free ligand shows a medium band at 1570 cm-1 shifting towards lower wave number is due to the coordination of azomethine nitrogen with metal ion. The proposed linkage with azomethine nitrogen with metal ions are further supported by the appearance of a band in for IR region at 435-460 cm-1 in the complexes assigned 26, 27 to nM–N. The >C=S band appearing at 840 cm-1 in IR spectrum of ligand is shifted towards lower wave number in the spectra of the complex. This is due to coordination of thione sulphur28 atom to metal ions. The linkage with metal ion with thione sulphur atom is further supported by the appearance of a band in far IR region at 395-410 cm-1 assigned26,27 to nM–S. The Coordination through halogen atoms are indicated by the appearance of a band in far IR region at 315-260 cm-1 assigned26,27 to nM–X(x=Cl–, Br–, I–). The coordination with halogen is supported by the low molar conductivity of the complexes in the range 8.9-14.3 ohm-1 cm2 mol-1. The band at 1320 cm-1 and 1440 cm-1 indicates mono-coordinate linkage of nitrate groups with metal ions. Linkage with oxygen atom of nitrate group is support by appearance of a far IR band at 545-515 cm-1 assigned to nM–O.
On the basis of IR spectral positions it may be proposed that ligand BTQT, acts as neutral bidentate ligand. Coordination proposes through azomethine nitrogen and thione sulphur to the metal ion. The remaining coordination centers are satisfied by negative ions such as Cl–, Br–, I– or NO3–.
Magnetic susceptibility electronic spectra
The electronic spectra of the Co(II) complexes exhibit three bands in the region 10600-9900 cm-1 16800-16100 cm-1 and 24200-23800cm-1 assigned to 4T1g(F) ® 4T2g(F), 4T1g(F) ® 4A2g(F) and 4T1g(F) ® 4T1g(P) respectively suggesting 29 an octahedral geometry of Co(II) complexes. The proposed geometry is further supported30,31 by the magnetic moment value in the range 4.86-4.99 BM.
The electronic spectra of Ni(II) complexes exhibit three bands in the region 11800-12300 cm-1 strong intense band in the vicinity of 24500 cm-1 assignable due to the transitions, 3A2g(F) ® 3T2g(F), 3A2g(F) ® 3T1g(F) and 3A2g(F) ® 3A2g(P) respectively. These positions propose29 an octahedral geometry for Ni(II) complexes which is further supported30,31 by the magnetic moment of value of Ni(II) complexes in the range 3.08-3.14 B.M. The electronic spectra pf Cu(II) complexes exhibit two spectral bands in the region 12900-13600 cm-1 and 19700-20300 cm-1 assigned to the transitions, 2Eg ® 2T2g and charge transfer band. The magnetic moment value of Cu(II) complexes lie In the range of 1.86-1.93 B.M. The above data of electronic spectra suggested an octahedral geometry for Cu(II) complexes.
Molar Conductance
Molar conductance of the complexes were measured in DMF at the concentration 10-3 and all the complexes were found to be non-electrolytic in nature due to 10 W value of molar conductance in the range 8.9-14.3 ohm-1 cm2 mol-1.
Antimicrobial Screening
Antibacterial activity of Schiff base ligand, BTQT and its Cobalt, Nickel and Copper complexes have been tested by disc diffusion technique33. The Gram positive and Gram negative organism such Gram negative bacterial E. Coli and Gram Positive bacteria Candida albicans were used to find out the antimicrobial activity. All the complexes showed a remarkable antimicrobial activity against bacteria. From the results it is proposed that the metal complexes are found to have more biocidal activity than the parent ligand.
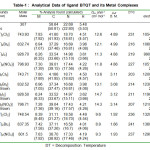 |
Table 1: Analytical Data of ligand BTQT and its Metal Complexes
|
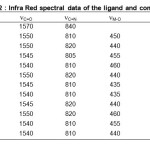 |
Table 2: Infra Red spectral data of the ligand and complexes. Click here to View table |
![Fig.1 [M(BTQT)2 ] M = Co(II) and Ni(II); X = Cl-, Br-, I- or NO3-. M = Cu(II); X = Cl-, Br- or NO3-.](http://www.orientjchem.org/wp-content/uploads/2014/07/Vol30_No3_Struc_Rai_fig1-150x150.jpg) |
Fig 1: [M(BTQT)2 ] M = Co(II) and Ni(II); X = Cl–, Br–, I– or NO3–. M = Cu(II); X = Cl–, Br– or NO3–. Click here to View Figure |
CONCLUSIONS
The IR spectral studies revealed that the ligand, BTQT acted as a neutral bidentate(NS donor) in all the complexes. The magnetic conductance and electronic spectral studies reveal the complexes were paramagnetic with octahedral geometries. The remaining valency are satisfied by negative ions such as Cl–, Br–, I– or NO3–. All investigated complexes were non-electrolytic in nature as given in Fig.-1.
REFERENCES
- Abbert A. and barlin G.B., J. Chem., Soc; 3129(1962).
- Ghorab M.M., El-Sayed B.S., Saker H.M. and Rabo M. Abd, Arzneimittelforsc, 56, 665(2006).
- Jessy E.M., Vachala D., Navneet K. and Srinivassan, Pharacology online, 2, 618(2008).
- Gupta V. kashaw S.K., jatav V. and Mishra Med. Chem. Res; 17, 205(2008).
- Hamed M.S., Kamel M.M., Kaseem M.M., Notal M.S. and Ahmed F.M. Acta Poloniae Pharmaceutica-Drug Res. 67, 159(2010).
- Al-obaid M., Abdel-Hameid S.G., El-kashet H.A., Abdul Aziz A.A.M., El-Azab A.S., al-Khamees M.A. and El-Subbagh H.I., Euro J. Med. Chem; 44, 2379(2009).
- Al Omar M.A., El-Azab A.S., El-Obeid H.A. and Abdul Hameide S.G., J. Saudi Chem; Soc; 10, 113 (2006).
- Kumar A. Sharma s., archana Bajaj K., Sharma S. Panwar H., Singh T and Srivastav V.K., Biooir.Med. Chem., 11, 5293(2003).
- Lu C., Yang J., Chiang J., Hour M, Lin K., Lin J., Huang W., Tsuzuki M., Leel T. and Chung J., Plosone, 7, 1(2012),
- Rai B.K., Sinha Puja, Vidhyarthi S.N. and Singh Vineeta, Asian J. Chem., 23, 4629(2011).
- Rai B.K., Singh Vineeta, Vidhyarthi S.N. and Sinha Puja, Asian J. Chem, 23, 4638.
- Rai B.K., Sinha Puja, Vidhyarthi S.N. and Sinha Puja, Singh Kalyan Chnadra, Sahi Shashi Bhusan and Ojha Jayvir Sharan, Orient J. Chem, 28, 1365(2012).
- Rai B.K., Vidhyarthi S.N., Amit, Singh Rabindra, Bharadwaj Nitish and Ojha avinash, Orient J. Chem; 28, 1403(2012).
- Rai B.K., J. Indian Chem; Soc., 90, 105(2013).
- Rai B.K., Vidhyarthi S.N., Kumari Punam, Kumari sunam, Kumari Lakshmi, and Singh Rajkishore, Asian J. Chem., 25, 941(2013).
- Rai B.K., Vidhyarthi S.N., Singh Puja, Singh Vineeta and Kumar Snajeev, Orient J. Chem., 29, 271(2013).
- Rai B.K., Kumar Sanjay, anand Rahul and Pandey ashok, Orient J. Chem, 29, 655(2013).
- Rai B.K., Singh Rabindra, Anand Puja, Singh Sunil, Kumar and Amit, Orient J, Chem, 20, 753(2013).
- Rai B.K., Vidhyarthi S.N., Prakash Om, Balunil Akhilesh, Orient J. Chem., 29, 801(2013).
- Choudhary Chitranjan Prasad, Sharma S.P., Rai C.L. and Rai B.K., Orient J. Chem., 29, 963(2013).
- Kumari Rachana and Rai B.K., Orient J. Chem, 29, 1163(2013).
- Rai B.K. and Kumar Arun, Orient J. Chem; 29, 1187(2013).
- Kumar Ajit, Yavadav U.S. and Rai B.K., Orient J. Chem., 29, 1203(2013).
- Vogel A.I., A Textbook of Quantitative Chemical Analysis, Revised by Besset J., Denny R.C., Jeffery J.H. and Mendham J., ELBS, 5th Edn., London(1996); Chaube S.N., Shrivastav J.P. and Mishra L.K., Inorg. Chem., Acta, 23,1(1977).
- Agarwal R.K., arora K., Polish J. Chem., 67, 219(1993).
- Singh N.K., Shrivastav A.K., Agarwal R.C., Indian J. Chem; 22A, 704(1984).
- ferraro J.R., Low Frequency Vibration of Inorganic and Coordination Compounds, Plenum Press, new York.
- Banerjee P., Singh I.P., Indian J. Chem., 6, 34(1968).
- Lever A.B.P., Electronic Spectroscopy, Elsevier, Amsterdam, 395(1968).
- Figgis B.N., Introduction to Ligand Field, Wiely Eastern Ltd., New Delhi, 279(1976).
- Carlin R.L., Van Dryneveldt A.J., Magnetic Properties of transition metal Compounds, Spriner Verlag, new York(1997).
- Kettle S.F.A., Coordination Compounds, Thomas nelson and Sons, 168(1975).
- Mukhurjee P.K., Saha K., Giri s.N., Pal M. and Saha B.P., Indian J. Microbiology, 35(1995).

This work is licensed under a Creative Commons Attribution 4.0 International License.









