Removal of Aluminum from Water and Industrial Waste Water
Parisa Ghashghaiee pour1, Mohammad Ali Takassi2*, Touba Hamoule2
1Department of chemistry, science and research branch, Islamic Azad University, Khouzestan, Ahvaz, Iran 6198144475
2Department of science, Petroleum University of Technology, Ahwaz, Iran 6198144471
DOI : http://dx.doi.org/10.13005/ojc/300356
Article Received on :
Article Accepted on :
Article Published : 15 Sep 2014
This study attempts to introduce a procedure to remove Aluminum ions from drinking water and industrial effluents by using active carbon with different grading as absorbent. Absorption of Aluminum ions were discussed in different conditions of Aluminum concentration, contact time, impact of electrolytes and pH on Aluminum ions absorbency. Both Freundlich and Langmuir isotherms used to investigate the adsorption. Thermodynamics relations governing process, such as specification of ( ), ( ) and the enthalpy of adsorption, were calculated, which showed that Aluminum absorption on active carbon is an endothermic and spontaneous process.
KEYWORDS:active carbon; adsorption; industrial effluents
Download this article as:| Copy the following to cite this article: pour P. G, Takassi M. A. , Hamoule T . Removal of Aluminum from Water and Industrial Waste Water. Orient J Chem 2014;30(3). |
| Copy the following to cite this URL: pour P. G, Takassi M. A. , Hamoule T . Removal of Aluminum from Water and Industrial Waste Water. Orient J Chem 2014;30(3). Available from: http://www.orientjchem.org/?p=4646 |
Introduction
Despite its range of applications, aluminum is one of the rare affluent elements which seemingly have no positive impacts on the living cells. At high quantity, it affects the blood circulation and nerve systems [1]. Aluminum would be absorbed more from food stuffs than water [2]. Studies have revealed that consumption of acidic food stuffs and liquids containing aluminum could result in considerable increment of aluminum absorbance [3]. Poly aluminum chloride (PAC) and aluminum sulfate are mineral coagulants which have been highly used in recent years for removal of color and turbidity in the water treatment industry [4-8]. Having been added to the water, a part of coagulants would be dissolved and remained in the final treated water. Aluminum is among elements existing in aluminum-based coagulants (e.g. PAC and Alum) that even its trivial amount in the water causes numerous health problems including Alzheimer and dialysis encephalopathy [9]. The maximum allowable aluminum extent in drinking water is set 0.2 Mg/L by the Environmental Protection Agency (EPA). Ideally, the aluminum extent in drinking water should be less than 0.05 milligram per liter and the maximum level allowed is 0.2 milligram per liter. Having access to safe drinking water is considered among the essential needs of any community. The population growth and development of cities, industries etc. have polluted the environment and supplying sources of drinking water in particular [10,11]. Preserving the quality of water in terms of aluminum level and controlling potential contaminations by resources had required the necessary planning and researches; aluminum extent was, therefore, measured on a very small scale while the attempt was simultaneously made to reduce or remove it from the industrial effluents [12]. In the present study, activated carbon was used to remove aluminum ions while aluminum absorbance under the conditions of differing aluminum levels, the contact time of aluminum with activated carbon, the effect of electrolyte on the absorbance level and the effect of PH on the aluminum absorbance were investigated. The related graphs were drawn to obtain the maximum absorbance efficiency. At the end, the thermodynamic parameters of the process were determined.
Methodology
Equipment
The following equipment’s were used:
UV-Vis Spectro photo meter Thermo SCIENTIFIC Company; Hot Plate, Genesis 10s model, IKA ; Mettler Scales, Jar Test, Lovibond Mode. l
Chemical materials
All solutions were made of deionized water with the electrical conductivity less than one micro Siemens. Tetrazole aluminum (aluminum(III) chloride salt in water, equivalent to 1000 micro grams aluminum) made by Merck Company, Germany, was used to produce the standard aluminum solution. Solutions of lower concentration were produced through the process of step-by-step dilution; activated carbon in mesh size of 20.
The method of measuring aluminum
The remaining aluminum was measured through aluminum measurement standard number 3500-A 1B (Eriochrome Cyanine R Method) using a wavelength of 535 nm.
Optimizing the condition
In order to achieve the best sensitivity, obtain the best condition for the segregation process and measure aluminum, it is required that all segregation conditions be examined separately. During the experiment, therefore, all agents but the one that should be changed were kept fixed and the changed agent’s impact on the segregation process was studied.
The concentration effect
To study this parameter, 50 milliliters of a solution having aluminum ions with the concentration of 20 to 5000 micrograms per liter was poured into one gram of activated carbon in mesh size of 20. It was mixed by a 360-rotation-per-minute (rpm) mixer for five minutes, the particles of activated carbons which had absorbed aluminum ions were separated by a filter paper and the filtrate was remained.
The initial aluminum extent was 20 to 5000 micro grams per liter and the remained aluminum extent subsequent to the segregation would be calculated through the following equation
Percentage removal of aluminum ion =
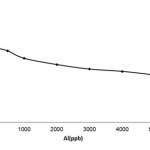 |
Figure1: Effect of aluminum ion concentration on adsorption by absorbent. |
Contact time effect
At this stage, the contact time between one gram of activated carbon and 50 milliliters of the solution having aluminum ions with the concentration of 3 milligrams per liter was changed between one to ten minutes. The contact time of five minutes with the mixing velocity of 360 rpm suffices the completion of the operation of aluminum removal (Fig. 2).
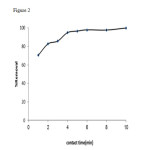 |
Figure2: Effect of time of contact on aluminum ion adsorption by absorbent Click here to View Figure |
pH effect
To study the pH effect on the process of removing aluminum ions, fifty milliliters of the solution containing aluminum ions with the concentration of three milligrams per liter were poured into a beaker containing one gram of the particles of the activated carbon. Subsequent to the measurement of the aluminum concentration, the removal extent would be calculated. The desired pH is set by 1 and 0.1 molar solutions of hydrochloric acid and sodium hydroxide. In order to achieve three milligrams concentration and the desired volume, the content of the beaker would be finally transferred to the volumetric flask. The results (Fig. 3) revealed that removal of trivalent aluminum ions would be more efficiently carried out in the range of pH 4-8.
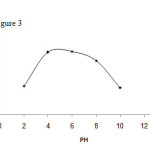 |
Figure3: Effect of pH changes on aluminum adsorption by adsorbent |
The effect of changing absorbent level on removal of aluminum ion
To optimize the absorbent level for removing aluminum ion from a fifty-milliliter solution (3mg/L); the experiment was carried out with different amount of absorbent.
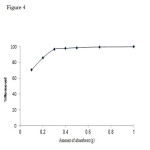 |
Figure4: Effect of the amount of absorbent on aluminum ion removal from solution. Click here to View Figure |
As Fig. 4 shows, the maximum aluminum removal occurs when 0.3 grams of activated carbon particles are used while higher quantities of absorbents do not positively affect aluminum removal percentage.
Removing aluminum ion from real samples
The accuracy and reliability of our measurements were confirmed by experiments with several water samples and the removal process was studied at optimized conditions. Real samples from karoon-river (a major river in southwest Iran) and waste water pool (Ramin-Ahwaz power station). The results are shown in Table 1 which reveals the possibility of applying the method to real samples without removing the matrix from the removal operation.
Table 1 the percentage of removal aluminum ion from water samples using activated carbon as absorbent.
|
Water samples |
Concentration of aluminum ion solution ppm |
Percent removal |
|
Karoon river |
70 |
99.8 |
|
Waste water pools (power plant) |
100 90 |
99.7 99.8 |
|
Waste water resulting acid wash of heat exchangers (power plant) |
100 |
99.6 |
Electrolyte effect
The effect of electrolyte concentration on removing aluminum ion from 50 milliliters aluminum solution (three milligrams per liter) was studied. NaCl 0.01-1 M solution was used as a strong electrolyte. The results revealed that the efficiency of removing aluminum under optimized experimental conditions is not affected by the electrolyte concentration.
Isothermal adsorption
To study the isothermal adsorption of aluminum ion on absorbent; The adsorption of aluminum ion on one gram absorbent were measured for samples with various concentration levels between 20 to 200 ppm. The Langmuir and Freundlich isotherm absorption graphs were plotted (Fig.5 and 6).
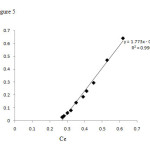 |
Figure5: Langmuir isotherm Click here to View Figure |
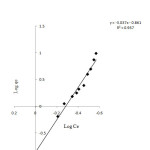 |
Figure6: Freundlich isotherm Click here to View Figure |
With regard to the values obtained for 0.990, the absorption is in line with Langmuir equation and the coatings on the activated carbon particles are monolayer.
Thermodynamic analysis of the absorption process
Thermodynamic parameters of ΔG, ΔS and ΔH are of very import in spontaneity and heat involved in the absorption process.
Kd=(Co-Ce) /Ce
Kd is the absorption distribution constant of the absorbent, Co is the primary concentration of the absorbent in mg/L and Ce is the equilibrium concentration of the absorbent in the solution in milligram per liter.
The ΔGo could be calculated by the following equation
ΔGo = -RTlnKd
(R is the gas constant (8.314 J/mol) while T is the temperature in Kelvin.)
Through the combination of the above mentioned equation and the following one
ΔG=ΔH-TΔS
The following equation would be obtained:
lnKd =ΔS/R – ΔH/RT
Enthalpy and entropy values could be calculated through drawing values of ln kd vs. 1/T.
In order to determine the impacts of absorption thermodynamic parameters on the absorbent, one gram absorbent was added to a one-hundred-millimeter volumetric flask containing standard solution of three ppm aluminum ion at a pH 5.5; the flask was, then, put on a 300 rpm magnetic mixer, the samples were instantaneously filtered and the aluminum ion existing in the solution was measured by the spectro photometer at a wavelength of 535 nm.
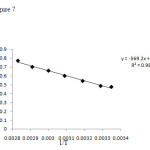 |
Figure7: Trend of temperature changes (meaning less) ???? Click here to View Figure |
As Fig. 7 shows, the process of aluminum ion adsorption on the activated carbon was spontaneous and endothermic in which the temperature increase would lead to the increase in the absorption distribution constant.
The process of calculating changes in ΔH, ΔS and ΔG
Conclusion
Absorbing aluminum ions from water solutions through extracting solid phase by activated carbon particles is considered one of the fast, simple and cheap methods. Less than five minutes is required for the completion of the absorption process and the process of aluminum absorption to the activated carbon is endothermic and spontaneous. The temperature increase would lead to the increase in the absorption distribution constant. The aforementioned method enjoys a high potential for removing aluminum ions with high efficiency from water and waste water.
References
- Banks, W.A.; Kastin, A.J. Neurosci. Biobehav. Rev . (1989), 13 , 47–53.
- Yokel R. A; Hicks C. L; Florence R. L . Food Chem. Toxicol. 2008, 46 , 2261–6.
- Slanina, P.; French, W; Ekström, L. G; Lööf, L; Slorach, S. Cedergren, A. Clin. Chem. 1986, 32 , 539–541.
- Chow , C. W.K; van Leeuwen, J.A; Fabris, R; Drikas, M. LENNTECH. 2009, 245, 120- 134.
- Ching-Jey , K; Gary L. A; Curtis, W. B. Water Res. 1988, 22, 853-862.
- Ghafari, S; Hamidi . A. A; Mohamed Hasnain, I; Zinatizadeh A .A . J. Hazard. Mater. 2009, 163, 650-656 .
- Baoyu. G; Qinyan. Y; Chemosphere. 2005, 61, 579-584.
- Sun, T; Sun. C. h; Zhu, G.li; Miao, X. j; Wu, C; Lv, S.B; Li, W.j. 2011, 268, 270-275.
- Ferreira, P. C; Piai Kde, A; Takayanagui A. M; Segura-Muñoz S. I. Rev. Latino-Am. Enfermagem. 2008, 16 , 151–7
- Joseph, A; Salvato, P.E,DEE,(1993),”Environmental Engineering and sanitation”, John Wiley, & Sons, Inc, New York, 4th Edition.; (1993).
- Rana. S.V.S., Environmental Pollution: Health and Toxicology, Narosa Publishing House. New Delhi, (2006).
- Karadjova, I.; Izgi, B; Gucer, S. Spectrochim .Acta. Part B. 2002, 57, 581-590.

This work is licensed under a Creative Commons Attribution 4.0 International License.











